Agricultural Biotechnology: What’s all the fuss about?
March 13, 2000
PAER-2000-01
Marshall A. Martin,* Professor and Associate Head
Frankenfood or silver bullet? The current debate over agricultural biotechnology is raging in the news media, in agribusiness boardrooms, among consumer and environmental activist groups, among government officials and international trade negotiators, within the scientific community, and in rural communities across this nation.
Biotechnology is truly ubiquitous. Everyone is impacted directly or indirectly by the products of biotechnology now flowing out of the laboratories, farms, and food processing plants throughout much of the world. How did this new science evolve? What has happened in recent months? What does it mean for society? And, where are we headed?
This article provides background on biotechnology, explores the expected benefits and possible risks associated with the products of bio-technology, and offers insights on potential current and future societal impacts.
The Scientific Foundation
Some say that biotechnology is nothing new. Mankind has domesticated plants and animals for thousands of years. Enzymes have been used since Biblical times to produce cheese, bread, beer, and wine. Gregor Men-del, in the mid-19th Century, established the basic laws of genetics that led to our understanding of inheritance traits and the role of genes in transferring these traits among off-spring of plants and animals. The ensuing applications of Mendelian genetics have resulted in hybrid corn, leaner hogs, more milk production per dairy cow, and the development of disease resistant crops. Thus, science has helped increase agricultural productivity.
But something is new today. Modern biology has its roots in the pioneering research by Nobel Laureates Drs. Watson and Crick. In the early 1950s, they unraveled the mystery of how the
“code of life” is transferred from one generation to the next through the spiral-helix. Their research helped us understand how deoxyribonucleic acid (DNA) functions as an information storage system that can be easily and accurately replicated. DNA is composed of four building blocks denoted by their nitrogenous base components: A (adenine), C (cyto-sine), G (guanine), and T (thymine). With the 26 letters of the alphabet, we use various sequences of letters to form words and sentences in English to store and communicate information. In an analogous fashion, these four nucleotides can be placed in various chemical combinations to store and communicate biological information. Most DNA molecules are extremely long strands of millions of nucleotides. Like a zipper on a jacket, these building blocks are placed in a double strand, which can be “unzipped” or rearranged to trans-fer genetic information.
Proteins do virtually all the work in the cells that contain the DNA. The cells use the information in the DNA to determine what the proteins should do. By inserting a new gene in the DNA, which occurs with genetic engineering, a new message can be sent to produce new proteins or modify existing proteins in the cell structure.
The techniques of molecular biology or biotechnology have made it possible for scientists to move genes into the DNA of one plant or organism from another plant or organism. The resulting plant or organism can be referred to as “transgenic”. Such a transfer of a gene would not be possible through classical genetics or plant breeding. It is the development of these techniques of gene transfer or genetic engineering that has given rise to new processes and products that are increasingly pervasive in medicine, food processing, and agriculture. (See Figure 1 for an example of transferring a gene from a soil bacterium that can express insect resistance in corn.)
Medical Applications of Biotechnology
One of the first major applications of biotechnology was the development of a genetically engineered version of insulin. For decades, companies like Eli Lilly in Indianapolis had purified insulin from the pancreas of hogs and cattle. Diabetics could inject this animal-based insulin on a regular basis to regulate their blood sugar. With a growing and aging population, and improved medical diagnosis, the incidence and cost of treating diabetes with animal-based insulin was increasing. Also, some people’s bodies reject these animal-based insulin products.
By taking the gene that codes for insulin from a person’s pancreas, and using the techniques of genetic engineering in a laboratory using appropriate E.Coli bacteria, Eli Lilly scientists were able to produce a pure copy of human insulin in large, relatively inexpensive quantities. After many years of careful testing, the Food and Drug Administration approved the commercial sale of Humalin® or Humalog® for the treatment of diabetes. This product of biotechnology is widely used by diabetics throughout the world.
Vaccines against many common diseases, human growth hormones to treat dwarfism in children, laboratory assays such as the home-pregnancy test, and many other applications of biotechnology are common in the medical community today. In addition, a whole new field of “farmaceuticals” is emerging as pharmaceutical products such as blood clotting agents are produced in goats’ milk.
Food Processing
The processing of many foods requires the use of enzymes to con-vert starches or proteins into desired end products. Examples include extracting fructose from corn, con-verting the starches in barley or grapes into sugar that can then be fermented to make beer or wine, or converting the proteins in milk into cheese curds.
Rennin has been used for centuries to produce cheese from milk. Rennin is found in the stomach of a veal calf. Using the process of genetic engineering, a version of rennin called chymosin has been produced in a laboratory. Today, nearly 80% of the cheese consumed in the United States is processed using chymosin.
Livestock Production
In the 1980s, four different companies, Cyanamid, Upjohn, Elanco, and Monsanto
conducted research on the development of genetically engineered Bovine Somatotropin (Bst). Bst, a protein hormone produced in the pituitary gland of dairy cows, is partially responsible for stimulating milk production in the mammary glands. Animal scientists have been aware of this process for many decades. It is now possible, using the process of genetic engineering, to extract from the pituitary gland of a dairy cow the gene that codes for Bst and then cheaply produce large quantities of Bst in a laboratory using E.Coli bacteria.
In the late 1980s, Monsanto successfully did this and then patented a method to inject Bst into a dairy cow on a monthly basis during the latter months of lactation. The commercialization of this product, under the brand name Posilac®, was launched in the United States in February 1994. Currently, about 15% of U.S. dairy farmers, who man-age about one-third the dairy herd, use this product. It can increase daily milk production by about 10 pounds per cow.
Bst was not launched without controversy, however. Critics claim that it was not adequately tested and might cause cancer in humans who consumed milk and dairy products from cows treated with Bst. Others were concerned that higher producing cows would have a higher incidence of mastitis requiring greater use of antibiotics which might remain in the milk and adversely affect consumers. Still others were concerned about the impacts on the cows’ health including possible reproductive problems. Despite these concerns, U.S. dairy farmers are using Bst, and none of the critics’ animal or human health concerns have emerged to date as serious problems. In fact, milk and dairy product consumption in the United States continues to increase.
However, in other countries, expressed health and economic con-cerns have precluded the approval of Bst use by government agencies. For example, Bst is not approved for use in the European Union (EU). Many EU consumer groups are opposed to biotechnology. Also, the EU’s Com-mon Agricultural Policy for several decades has imposed a marketing quota system on its dairy farmers that limits milk sales to avoid sur-pluses of butter, cheese, and other dairy products.
Research continues on other ani-mal applications of biotechnology including Porcine Somatotropin (Pst) to enhance lean muscle growth and reduce fat deposition in hogs. The development of vaccines to control animal diseases also continues.
There are potential animal applications to human medicine. For example, research is underway in what is called xenotransplants. Scientists are exploring the possibility of transplanting animal organs, such as a pig heart, into humans. While this may become a cost-effective way to save human life, it also raises critical ethical questions.
Crop Biotechnology
Weed and insect control has been a challenge for farmers for centuries. Following World War II, pesticides were developed and rapidly adopted by farmers. While most were safe and effective if used properly, some were not, and in isolated cases were removed from the market.
Genetic engineering offers a way to alter crops to resist insect pests or become tolerant to less toxic and environmentally safer herbicides. In 1996, Monsanto, and affiliated seed companies, launched the commercial sale of Round-Up Ready® soybeans. By 1999, 57% of the U.S. soybean acreage was planted to soybean varieties with this herbicide-tolerant trait. Compared to the soil incorporation of some conventional herbicides, this seed technology encourages no-till farming practices, which can help reduce soil erosion and water pollution. The gene that encodes tolerance to the herbicide glyphosate (Round Up®), using the techniques of genetic engineering, was transferred from Agrobacterium sp. strain CP4, a soil bacterium. The gene is a single dominant gene and is stable over several generations.
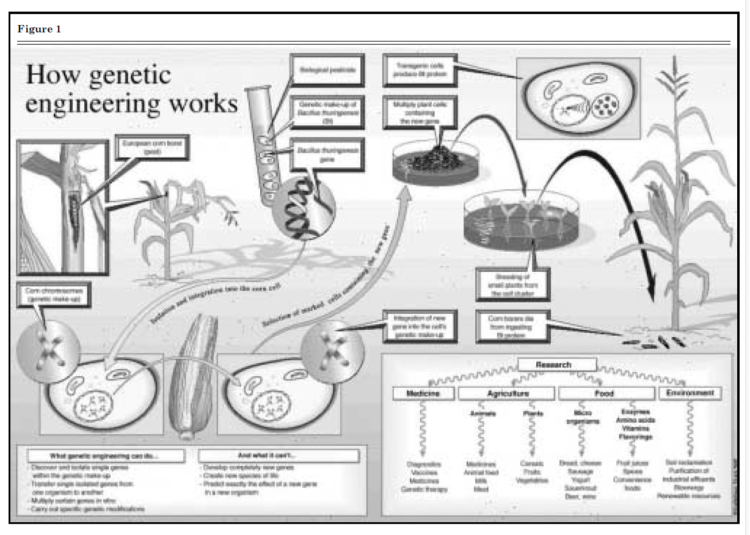
Figure 1. How Genetic Engineering Works
Bacillus thuringiensis (Bt) is a soil bacterium that has been used for several decades by gardeners and organic farmers to control insects. Using the tools of genetic engineering, scientists have inserted the gene that codes for this protein into several crops including corn, cotton, and potatoes. The crystalline protein has a complex molecular structure. This allows scientists to select the specific molecular structure that targets a specific insect such as European corn borer in corn, pink budworm and boll weevil in cotton, and Colorado potato beetle in potatoes. Once the target insect ingests a few bites of the plant tissue that contains the Bt protein, the insect’s digestive system converts the protein into a toxin that destroys the cell membrane of its stomach and kills the insect. However, when an animal or a human consumes the Bt protein in the plant, the acid environment of the stomach promotes digestion of the protein, without any toxic effects.
In 1999, about one-half the cotton and one-third of the corn acreage in the United States was planted to transgenic varieties. This has resulted in a reduction in insecticide use, primarily for cotton. However, since the high-dose strategy of insect management, if widely adopted, could place extreme pressure on the target insect population, insect resistance management (IRM) programs are essential to minimize the development of insect resistance to Bt. In January 2000, the U.S. Environmental Protection Agency approved a refuge management strategy for corn that requires Midwestern farmers to plant a 20% refuge to non-Bt corn varieties within one-quarter mile of the Bt corn. Entomologists have determined that resistance to Bt is a recessive trait. If some European corn borer survive in the 20% refuge portion of the field and mate with those adults in the portion of the field planted to Bt corn, it is expected that a viable number of European corn borer will survive without the recessive trait, and insect resistance to Bt will be at least delayed, if not avoided.
Besides the input traits such as insect-resistance and herbicide-tolerance, a number of output traits are being introduced into crops. Through genetic engineering, scientists have added vitamin A into rice. Rice is the primary food grain eaten as a staple in the diets of mil-lions of people, especially in the developing world. World health experts hope that these vitamin A enhanced rice varieties will reduce by about 500,000 the number of people who go blind each year and by 2 million the number of children who die each year due to vitamin A deficiency.
Other examples of output traits include phytase in corn that increases the availability of phosphorous in hog rations and reduces the amount of phosphorous added to the feed premix. With greater utilization of phosphorous in the hog’s digestive system, there is less phosphorous in the hog manure and a reduction in the amount of phosphorous applied to fields. This should help reduce hog production costs and offer an environmental benefit.
“Farmaceuticals” such as genetically engineered tobacco to produce cancer-treating drugs are under development. Also crops such as bananas and potatoes have been engineered to deliver selected vac-cines against childhood diseases.
The development of output traits through biotechnology will require producers to follow strict identity preserved practices including cultural practices, careful cleaning of harvesting equipment, and separate storage facilities. Those who are able to do this effectively should expect to receive a premium for adding value to the product.
Who regulates biotechnology?
Environmental quality and food safety are critical issues in the minds of many people. People won-der if these genetically engineered crops will have an adverse impact on biodiversity, water quality, or soils. Others won-der if these genetically engineered foods are safe to eat and if they might have some undesirable long-term impact on human health.
To address these concerns, the United States has three major regulatory agencies—The United States Department of Agriculture (USDA), the Environmental Protection Agency (EPA), and the Food and Drug Administration (FDA). Under Federal law each agency has specific regulatory authority.
The Animal and Plant Health Inspection Service in the USDA has oversight responsibility for the movement of seed across state lines. It also must authorize field-testing of any genetically engineered crops. Researchers, whether in the private sector, government laboratory, or university setting, must obtain approval before initiating any field-testing. Scientific peer review of the proposed research protocol is essential. This protocol must clarify the purpose of the research, how and where it will be conducted, what data will be gathered, how the test site will be monitored and secured, and how any crop residue will be dis-posed. Such reviews can take many months, and sometimes years, depending on the environmental and scientific issues associated with a specific field experiment.
The EPA has the authority to regulate pesticides and the environmental impacts of biotechnology discoveries. For example, by genetically engineering the Bt trait in corn, cotton, and potatoes, these crops technically become a pesticide and thus under the purview of the EPA. Hence, the EPA carefully examined how these Bt crops might impact the environment prior to their approval. One concern was the potential development of insect resistance if these insect-resistant crops were widely adopted by farmers. Because of this concern, in January 2000 the EPA mandated that any farmer growing Bt corn in the Midwest must plant a 20% refuge to a non-Bt corn variety to minimize the possibility that European corn borers develop resistance to the Bt trait. Insect resistance to Bt is a concern to farmers and agribusiness firms since this new bio-engineered crop might quickly be lost as a means of insect control. Also organic farmers and gardeners fear the loss of the effectiveness of traditional Bt insecticides if Bt crops are widely adopted with-out an effective refugia strategy.
The FDA has responsibility under the Food, Drug, and Cosmetic Act to regulate food and feed, as well as human and animal health products. For example, the FDA made two rulings on the use of Bst—one to approve its safety for human consumption and the other on safety for the treated dairy cows. This process took more than a decade, with approval for human safety in 1986, and approval for commercialization to U.S. dairy farmers in 1994. The FDA has reviewed and approved other products of biotechnology such as Bt in cotton, potatoes and corn; Round Up Ready® soybeans; chymosin for cheese production, and several medical applications including treatments for diabetes, arthritis, hepatitis, cystic fibrosis, and several types of cancer.
A European Perspective
Much of the recent anti-biotechnology resistance or concern has come from the European Union. This has impacted multilateral trade and biosafety negotiations, international trade, research investments, intellectual property rights, and marketing decisions by major corporations, and intergovernmental agency relations.
There are several factors that have contributed to this trans-Atlantic controversy. There has been a strong “green” movement in Europe for several decades. Some environ-mental groups historically have focused on point and nonpoint pollution issues ranging from oil spills in the North Atlantic to confinement livestock feeding and field applications of animal wastes in Western Europe. Recently, organizations such as Greenpeace and Friends of the Earth have played a very active role in preventing the EU from importing Genetically Modified Organisms
(GMOs) such as Bt corn and Round Up Ready® soybeans. They have also influenced European government officials to ban the planting of GMO crops by European farmers.
Lack of a credible EU-wide regulatory system appears to limit public trust in these new technologies and food products. The European Union has made considerable progress in recent decades to form a multi-country system of government with a Parliament, regulatory agencies in Brussels, a common currency (Euro), the elimination of national passports to cross EU borders, and the liberalization of intra-European trade. However, much of the food related regulatory responsibility in Europe remains at the national level. While efforts are underway to create a European-wide, coherent regulatory system related to biotechnology and the food and agricultural system, no such coherent regulatory system exists yet.
Several recent food scares have further eroded European confidence in their regulatory system. These scares include the mishandling in Great Britain of the “mad cow” dis-ease, dioxin contamination of live-stock feed, and the reported contamination of Coca-Cola® products in Belgium.
This lack of public trust in food regulation and oversight agencies has caused some Europeans to won-der if the products of biotechnology have been adequately tested and reviewed by European authorities. Some Europeans appear to be reluctant to rely too heavily on biotechnology products produced and regulated by U.S. companies and agencies.
When consumers purchase a product, they generally are seeking an actual, or at least perceived, bene-fit. Some European consumers are not convinced that there is any bene-fit from consuming bio-engineered products. With the current input-trait oriented biotechnology crops such as Bt corn or Round Up Ready® soybeans, there is no claim that foods based on these crops pro-vide any nutritional or health bene-fit. In fact, the FDA uses the “substantially equivalent” criterion to judge biotechnology food products as safe when there is no difference in their chemical or nutritional makeup from conventional foods. Moreover, under the EU’s Common Agricultural Policy, imports of U.S. corn are subject to a variable import tariff, which results in no price advantage to European consumers even if U.S. production costs and prices decline as U.S. farmers adopt Bt corn. Consequently, it is unlikely that these bio-engineered crops will cost Euro-pean consumers less. And, given the slightest doubt in European consumers’ minds about the food or environ-mental safety of these products, it is understandable why many Europeans are reluctant to purchase GMO-foods, and why they want some type of labeling system that allows them to select non-GMO foods if they wish.
European attitudes do vary by country and application of biotechnology, however. There tends to be the least support for biotechnology in the Germanic countries (Germany and Austria) and Scandinavian countries (Denmark and Sweden), but less opposition in the Mediterranean countries (Spain, Portugal, and Greece). About three-fourths of the Europeans who responded to a recent Eurobarometer survey sup-ported using biotechnology to detect and treat human diseases. However, there was much less support (one-half or less) for food uses or xenotransplants of organs from animals to humans. Many felt the risks were too high, and had moral objections to the food and organ trans-plant applications of biotechnology.
American Attitudes
Americans appear to be much more supportive of bio-technology than Europeans. In an October 1999 sur-vey, two-thirds of the consumers favored foods produced using bio-technology and expressed confidence in the current FDA food labeling policies. In fact, about 80% of the respondents indicated that they would prefer a toll-free number or a website rather than a food label about the biotechnology ingredients of a food that they might purchase. However, there is pressure from some consumers for food companies to provide more information about bio-engineered foods.
This recent U.S. consumer survey also reported that three-fourths of the respondents knew something about biotechnology, and two-thirds expected to benefit from biotechnology in the next five years. More than two-thirds indicated they would purchase food products enhanced through biotechnology that protected crops from insect damage and required fewer insecticides. How-ever, only 40% of the respondents knew that many food products in grocery stores today are genetically engineered.
Asian Experience
Asian countries, especially Japan, are major markets for U.S. agricultural exports. Asian countries currently purchase nearly two-thirds of U.S. corn exports and nearly one-half of U.S. soybean exports. With economic recovery in Southeast Asia from the 1997-99 financial crisis, the potential admission of China into the World Trade Organization (WTO), and expected population and per capita income growth in this region of the world, Asian imports of U.S. agricultural products are essential to the eco-nomic health of U.S. agriculture. Adverse attitudes in Asian countries towards biotechnology could have a significant negative impact on U.S. agricultural exports to Asia, and thus U.S. farm income.
Public acceptance survey data on this region are more limited than for the United States or Europe. How-ever, the available data suggest that while most Japanese are not significantly concerned about agricultural biotechnology, many Japanese food importing and processing firms are seeking to purchase non-GMO commodities to satisfy the demands of their consumers.
World Negotiations
The WTO meeting in Seattle in December 1999, and the Biosafety Protocol meeting in Montreal in January 2000 both focused on several biotechnology issues. The current WTO Agreement under the Sanitary and Phytosanitary (SPS) guidelines requires a scientific basis for ban-ning the importation of a commodity for health or food safety reasons. To date, Round Up Ready® soybeans and most of the Bt corn varieties being grown in the United States have been approved by the appropriate government regulatory agencies in the United States, the European Union, Canada, Japan, and else-where. Hence, since these are all WTO member countries, any import restrictions on GMO products would need to be science-based, i.e., raise human, animal, and/or environmental safety concerns.
The “Precautionary Principle” agreed to in Montreal allows a country to block imports of GMO commodities on a “precautionary” basis in the absence of sufficient scientific evidence about their safety. Some are concerned that the “Precautionary Principle” will encourage Euro-pean governments to “protect” their inefficient farmers on the pretext of protecting consumer health. Current WTO sanitary and phytosanitary guidelines require a clear scientific basis to restrict trade of a product that might be hazardous to human or animal health. The agreement reached in Montreal appears to be a “messy compromise.” The underlying issue is how to reconcile different attitudes among countries about the risks of technological change without disrupting trade. Should biotechnology move forward unless it is shown to be dangerous, or should it be banned until it is proven safe?
The Biotechnology Critics
The critics of biotechnology raise several issues. Some lack trust in regulatory agencies and feel the regulatory agencies lack the personnel and funding to adequately review the stream of new biotechnology products flowing from the private and public research laboratories. Some anti-biotechnology groups worry about potential adverse long-run impacts of genetically engineering. Often they will mention past experiences such as DDT, mad cow disease, thalidomide, or Chernobyl, where scientists said something was safe and then new information or experience was to the contrary. Hence, part of the debate is about how much testing and study is sufficient to assure the public of the long-term safety of the application of biotechnology to our agricultural and food system.
Those who are concerned about food safety worry that a transgenic gene might behave in some unexpected way and cause an allergic reaction or result in a serious dis-ease. Still others are concerned about adverse environmental consequences such as pollen drift from an herbicide-tolerant crop that might result in a “superweed” that will be difficult to control in the future. Still others worry about insects developing resistance to a pest-resistant crop, and subsequently losing the use of a “bio-pesticide” such as Bt. This is especially a concern among organic farmers and gardeners.
Potential adverse impacts of Bt crops on beneficial insects concern many people. For example, a pre-liminary laboratory study by entomologists at Cornell University suggested that corn pollen on milkweeds would kill Monarch butterfly larvae. Subsequent field research by several different scientists indicates that the damage to the Monarch butterfly is minimal since the time of the hatch of the larva and corn pollination often differ, corn pollen does not normally drift very far outside the field, and most Monarch butterflies tend to lay eggs in weedy areas outside the corn field.
The Monarch butterfly case illustrates the importance of careful research and monitoring of crops that are bio-engineered to resist insects. It is critical to verify the impact of insect resistant crops on non-target insects, and inform the public of their impact. If there are undesired consequences, then adjustments must be made in the management of the technology, if approved, such as the refuge requirement for those producers who grow Bt corn. And, if there are potential severe environmental consequences, then the EPA or other appropriate regulatory agencies should not approve the technology.
Other groups raise ethical questions, especially about xenotransplants or cloning of animals. Still others question the control that a few major multinational companies might have over the world’s seed and germplasm. And with increased patenting of biotechnology products, this could reduce farmers’ independence to purchase inputs and sell their products. Hence, part of the debate is about how the structure of agriculture may change and become more concentrated on a global scale. Some fear further erosion of the family-based farm structure. Biotechnology, along with other economic forces, is encouraging the development of a large, supply-chain oriented global food system.
The U.S. Food Processing Sector Response
Given the uncertainty about consumer response in North America, and especially in Europe, several major food processors have indicated that they will not use GMO-crops in their foods. Examples include A.E. Staley, National Starch, Gerber, Heinz, and the Frito-Lay corn chip division of Pepsico.
Others that primarily process soy-beans or corn for livestock feed use in the United States continue to accept both GMO and non-GMO corn and soybeans. Some grain elevators also are willing to accept corn and soybeans without segregation.
Other firms such as Consolidated Barge and Grain that ship to foreign markets, especially from their Ohio and Illinois River terminals, have asked farmers to segregate their crops. This requires farmers to separate the crop at planting, harvest, and during storage and trucking. Some export-oriented companies have offered premiums of 10 to 20 cents per bushel for non-GMO soy-beans and about 10 cents per bushel for non-GMO corn. Expectations for the fall 2000 crop is that it will be a mixed market situation with some firms seeking identity-preserved corn and soybeans with a modest premium, while others will co-mingle the crop and not offer any premiums. The key is whether the crop is for food processing, export, or livestock feed use. Currently, about one-fifth the U.S. corn crop is exported, about one-fifth is processed for food and industrial uses, and the remainder is primarily for livestock feed.
Farmers Choices
U.S. farmers currently face some difficult crop management choices- whether to plant or not to plant transgenic seeds. To date, it appears that the acreage planted to Round Up Ready® soy-beans for the 2000 crop year will be similar to 1999. Round Up Ready® soybeans help farmers reduce tillage operations in many instances, pro-vide more flexibility to spray and manage weeds, offer yields comparable to non-Round Up Ready® soy-beans, and can result in cleaner fields during harvest with fewer price discounts due to foreign matter in the soybeans.
It is likely that about 55-60% of the U.S. soybean acreage will be planted to Round Up Ready® soy-beans again this year. In Argentina, it is estimated that at least 70% of the soybean area is planted to Round Up Ready® varieties.
There appears to be a modest export market for non-GMO soy-beans for selected Asian and Euro-pean buyers. The premium will likely be 10 to 15 cents per bushel, and perhaps more for selected food grade soybeans. There are recent reports that some European buyers are contracting non-Round Up Ready® soybeans in Brazil and are paying about a 12% premium per ton.
“Strip tests” are a fairly reliable indicator as to whether a load of soy-beans is Round Up Ready® or not. These tests are relatively quick and inexpensive. European buyers seem to want one percent or less “contamination” from GMO soybeans. Some Asian buyers may accept up to five percent “contamination”. More accurate PCR tests take longer and cost several hundred dollars per sample.
Deciding whether to plant trans-genic corn for the 2000 crop year is a more difficult decision. First, there are several Bt products on the market with slightly different efficacies for European corn borer control, and thus a difference in the technology fee per unit of seed. Several herbicide-tolerant corn varieties are avail-able such as Liberty Link® and Round Up Ready® corn. In addition, there are limited supplies of stacked genes with both the insect-resistant and herbicide-tolerant traits.
First, farmers must determine if historically there has been frequent European corn borer damage. In most of Indiana, the probability of economic damage from European corn borer is about 25% (one in every four years). In this case, the technology fee is greater than the expected returns from planting Bt corn. How-ever, with higher expected yields and/or prices and about a 40% probability of European corn borer damage a farmer can benefit. Of course, the farmer must also take into account the economic impacts of planting a 20% refuge that may result in lower yields on that portion of the field, plus a modest amount of extra labor at planting time to change seed in the planter. (See ID-219, Economics of Bt Corn at http://www.agcom.Purd ue.edu/AgCom/Pubs/agecon.htm#8)
Once the basic agronomic and associated economic costs and benefits have been analyzed, the farmer must also determine if there will be a market for transgenic corn in his area. For livestock feed, and some export markets, transgenic corn will be acceptable. However, some corn processors and some export buyers do not want GMO-corn. In some cases, they may pay a small premium (5 to 10 cents per bushel) for non-GMO-corn. If a farmer decides to grow both, then he must carefully segregate the crop in the field to minimize potential for pollen drift. In addition, combines, trucks, grain dryers, storage facilities, dump pits, etc. must be carefully cleaned to avoid co-mingling of GMO and non-GMO corn. Given the expected modest premiums for non-GMO corn, each producer will need to carefully determine if the expected premium is sufficient to cover the extra labor and handling costs that he might incur to segregate his corn. The Indi-ana Crop Improvement Association will provide farmers with a certification service. (For more information, call (765) 523-2535 or see http://www.indianacrop.org)
Summary
Agricultural biotechnology offers considerable promise to help farmers enhance productivity, add-value through identity preserved crops, alter the composition or nutritional attributes of foods, control insects, reduce pesticide use, prevent crop and animal diseases, enhance livestock productivity and product quality, and pro-vide novel means to produce
“farmaceutical” products.
Yet, biotechnology, like many previous technologies, must be carefully managed. Refuge management will be essential with the various insect resistant crops to prevent insects from becoming resistant to the trans-genic crop. Food must be carefully regulated, and, some type of science-based labeling may be necessary to assure consumers that the foods are safe and do not contain ingredients that might result in adverse health effects such as allergenicity.
Developing countries continue to seek ways to increase the productivity of their farm sector, which often operates in a harsh environment due to lack of rainfall, disease and insect pressures, or hard to manage soils. And, most of the population growth, and potential per capita income growth, is in the developing world, especially Asia and parts of Africa and Latin America. If appropriately adopted and managed, selective applications of biotechnology can help these nations improve the quantity and nutritional quality of their food supply.
The challenge is gaining public understanding and maintaining credible regulatory authorities to assure the public that the application of biotechnology will not have adverse environmental or health impacts. The key for acceptance of biotechnology will be to create regulations that the public trusts and products that deliver benefits consumers can see or taste.
Biotechnology Websites
The following websites offer a wide range of information and views on agricultural biotechnology.
http://www.agecon.purdue.edu/exten sio/biotech.htm
http://www.agry.edu/com/chatchew.h tm
http://www.biotech-info.net/index.ht ml
http://www.cast.science.org/biotc_ip. htm
http://www.consumersunion.org/food/food.htm
http://vm.cfsan.fda.gov/~lrd/biotechm .html
http://www.greenpeace.org/~geneng/http://www.extension.iastate.edu/feci /HGMO
http://ificinfo.health.org/
http://www.isb.vt.edu/
http://www.monsanto.com/
http://www.seeds.novartis.com/http://www.pioneer.com/usa/gmo/def ault.htm
http://www.ucsusa.org/agriculture/in dex.html
Http://www.usda.gov/biotechnology/
* The author wishes to express special appreciation to Christy Welch Stair, Jeffrey Hyde, Berdine R. Martin, Elaine Lipscomb, Phil Paarlberg, and Wally Tyner for helpful review comments.
