Xing Liu Lab
Department of Biochemistry, Purdue University
ABOUT US
Ubiquitination controls the stability, interaction or activity of numerous key regulatory proteins in eukaryotic cells. Using cultured human cells and model plants, the Liu lab aim to understand the mechanism of protein ubiquitination and how this post-translational modification determines proper cellular and organismal functions.
CONTACT US
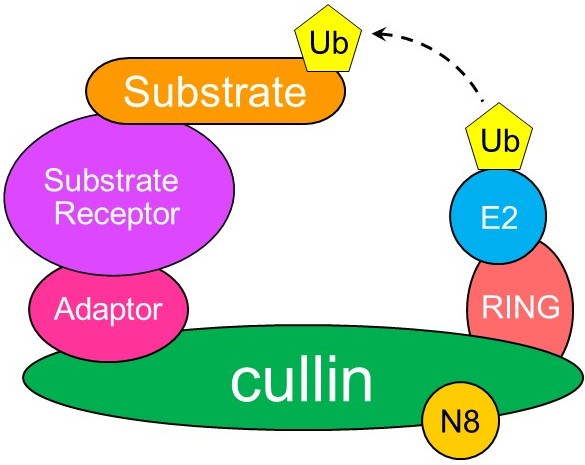
Composition of a cullin-RING ligase. The cullin-RING complex forms the enzymatic core. The substate receptor and adaptor form the substrate receptor module. Ub: ubiquitin. N8: Nedd8, a ubiquitin like protein that modifies cullins.
Our Research
Ubiquitination, the post-translational attachment of ubiquitin or ubiquitin chains, controls the stability, interaction or activity of numerous key regulatory proteins in eukaryotic cells. At the core of the ubiquitination process is the E3 ligase, which brings ubiquitin and the target protein together, and enables the transfer of the ubiquitin to its target. The Liu lab investigates the largest family of E3 ligases, known as cullin-RING ligases (CRLs). CRLs are modular protein complexes, featuring a common cullin scaffold and an interchangeable substrate receptor that recruits specific target proteins for CRL-dependent ubiquitination and subsequent degradation. We use a variety of approaches including biochemistry, biophysics, molecular genetics, quantitative proteomics, and mathematical modeling to study how CRLs work, how their activities are regulated, and what critical roles they play in cells and organisms.
Funding
Research projects in the Liu lab are currently funded by National Institute of Health (NIH) and American Heart Association (AHA). We are looking for highly motivated, independent, and organized scientists to join our team. Feel free to Contact Us for more information.