Adjuvants And The Power Of The Spray Droplet

Fred Whitford, Coordinator, Purdue Pesticide Programs
Gregory Lindner, Research Director, Croda, Inc.
Bryan Young, Associate Professor of Weed Science, Purdue University
Donald Penner, Professor of Weed Science, Michigan State University
Jason Deveau, Application Technology Specialist, The Ontario Ministry of Agriculture, Food and Rural Affairs
Douglas Linscott, Product Development Chemist, Dow AgroSciences, LLC
Heping Zhu, Agricultural Engineer, USDA Agricultural Research Service
Richard Zollinger, Professor of Weed Science, North Dakota State University
Eric Spandl, Technical Marketing Manager, Winfield Solutions, LLC
Bill Johnson, Professor of Weed Science, Purdue University
Kiersten Wise, Associate Professor and Extension Specialist, Purdue University
Aaron Patton, Assistant Professor, Turfgrass Extension Specialist, Purdue University
Chuck Champion, President, KALO, Inc.
Nick Harre, Graduate Research Assistant, Purdue University
Norman Wagoner, Business Development Specialist, Agrilead, Inc.
Kevin Leigh Smith, Editor, Purdue Agricultural Communication
The Perfect Team Play 4
Unleashing a Pesticide’s Potential 6
How Water Affects Pesticide Performance 8
Understanding the Properties of Spray Droplets 10
The Properties of Water Molecules 11
The Properties of Pesticide Molecules 14
Leaf Surfaces Affect Pesticide Performance 17
Considerations for Mixing Pesticides 21
Understanding Application Equipment 24
Adjuvants — Overcoming Water-induced Barriers 28
Adjuvant Classification and Nomenclature 30
Some Adjuvant Products Contain More Than One Adjuvant Type 42
The Pesticide Label: Suggestions and Silence 44
Buying Adjuvants With Confidence 50
Conclusions 57
Acknowledgements 58
Disclaimer 58
The Perfect Team Play
Leading by one run in the bottom of the ninth, the catcher calls for a fastball on the low outside corner of the plate. The pitcher throws a strike to win the game. With seconds to spare on the clock, the quarterback tosses a perfect spiral to his receiver who slips past the defender in the corner of the end zone for the winning touchdown. The wing-back passes the ball to the striker who kicks the ball just out of reach of the goalkeeper’s hands to help her team win an overtime thriller. The difference between winning and losing in sports often occurs when a team completes a single, well-executed play. When the game is over, everybody will talk about the star player’s perfect pitch, pass, or kick. But executing that perfect play was the result of preparation and teamwork. At every level, success in sport doesn’t rely on luck, but on planning, execution, and teamwork. In many ways, the perfect pesticide application is like that single, perfectly executed play. Pesticide applications and sports play both require preparation, knowledge, basic skills, and commitment. Both also require teamwork. The chemicals you mix in your tank have to work together — both the pesticides and the adjuvants, which are products that enhance pesticide performance. Applicators need to be on top of their games if they are to win the battle against the insects, weeds, and diseases that are the formidable opponents in successful crop production. Of course, one can only take the analogy between sports and pesticide application so far. Protecting health, property, crops, environment, and income is definitely not a game. Pesticide applications affect people’s livelihoods, and can lead to costly outcomes when the pesticide fails to control the targeted pests. And for these reasons, every application must be the winning play. This publication examines how the various components of a pesticide application work (water, pesticide molecules, application equipment). It then describes how adjuvants can enhance pesticide performance and protect against the unwanted consequences of spray drift. The goal is to define what adjuvants do and highlight their value in improving pesticide applications. This publication is a companion to The Impact of Water Quality on Pesticide Performance (Purdue Extension publication PPP-86), available from the Education Store, edustore.purdue.edu.

Unleashing a Pesticide’s Potential
Why does a pesticide work well one time and not so well the next when everything seems the same? Variable weather could play a role. Or the pest could be less susceptible to the active ingredient. At other times, an inconsistent pesticide performance seems inexplicable. In many ways, pesticide performance is similar to the way a seed corn hybrid’s yield varies from one year to the next or within a given field in the same year. The genetic pedigree of that hybrid determines its yield potential, but just because a hybrid has built-in potential doesn’t necessarily mean it will produce the highest possible yields. Performance potential is useful when discussing pesticides, too. Every pesticide molecule in a jug or bag has the built-in potential to control the pests listed on its label. You can maximize performance consistency when you appropriately match and time the right pesticide to the targeted pests, mixed at the labeled rate, and applied with calibrated equipment under favorable environmental conditions. However, even if you make every attempt to account for these factors, pesticides can still fail to produce expected or consistent outcomes. For example, a pesticide may not perform as planned because the pest developed resistance to it. But there are other factors that could have affected the pesticide’s performance. An important factor is the water in the spray solution. This will be our focus: conditioning the water that will carry the pesticide and modify the droplet characteristics can often improve spray quality and potency, which increases pesticide performance and consistency.
Water is the most common carrier of pesticide spray applications. A pesticide application to plant foliage relies on pesticide molecules in water droplets to transfer to the intended target pest.
The process of transferring an effective pesticide dose includes:
• Selecting the right product
• Calculating the rate
• Measuring the product accurately
• Uniformly applying the spray mixture with an appropriately set up and calibrated sprayer under favorable environmental conditions
Pesticide applicators often ignore (or underappreciate) water quality.
Poor water can lessen the effectiveness of the pesticide in the spray mixture, influence spray quality, and affect how the pesticide interacts with the atmosphere and the leaf surface.
Here are some examples of water quality’s effect on pesticide performance:
• Certain pesticide molecules can be deactivated when they bind with cations (positively charged molecules) in the water such as calcium (Ca+), magnesium (Mg+), and iron (Fe+). Sodium (Na+), manganese (Mn+), and potassium (K+) are significant in some regions.
• Spray water that is too acidic or too alkaline may degrade some pesticides or make them less soluble in the mixture.
• Smaller spray droplets may evaporate after exiting the sprayer nozzle before they even reach the target.
• The wind can blow smaller spray droplets away from the intended target (this is called spray drift).
• Spray droplets can bounce, shatter, or roll off the leaf surface (a lack of spray retention).
• Spray droplets that remain on the leaf surface may form a bead and fail to spread.
• The deposit formed after the spray evaporates may not have the right chemistry or enough active ingredients present.
• The active ingredient contained in the spray residue on the leaf surface may fail to penetrate the leaf.
The common denominator among these multiple fail factors is that they are all linked to the molecular, chemical, and physical properties of water in the final spray mixture. Spray deposit retention on the leaf and the chemical composition of the spray droplet will ultimately determine the effectiveness of the pesticide application.
To make more effective applications, it is important to understand what makes up a spray droplet and how
they work.
Understanding the Properties of Spray Droplets
Understanding water chemistry and how it interacts with the intended target surface is as fundamental to a successful application as selecting the right pesticides and managing application equipment. Typically, water is inexpensive, convenient, and safe to plants. While water’s properties generally make it an excellent carrier for most pesticides, it doesn’t necessarily mean that water alone is the perfect carrier for all applications.
Understanding how spray adjuvants and pesticide formulations influence the physical and chemical properties of water is more than an academic exercise. The properties of water in the spray solution affect how spray droplets form and influence how the application equipment applies them. You may have to modify water’s properties, spray quality, and droplet size to achieve the desired efficacy.
This section describes some of the important physical and chemical characteristics of water and of pesticides.

The Properties of Water Molecules
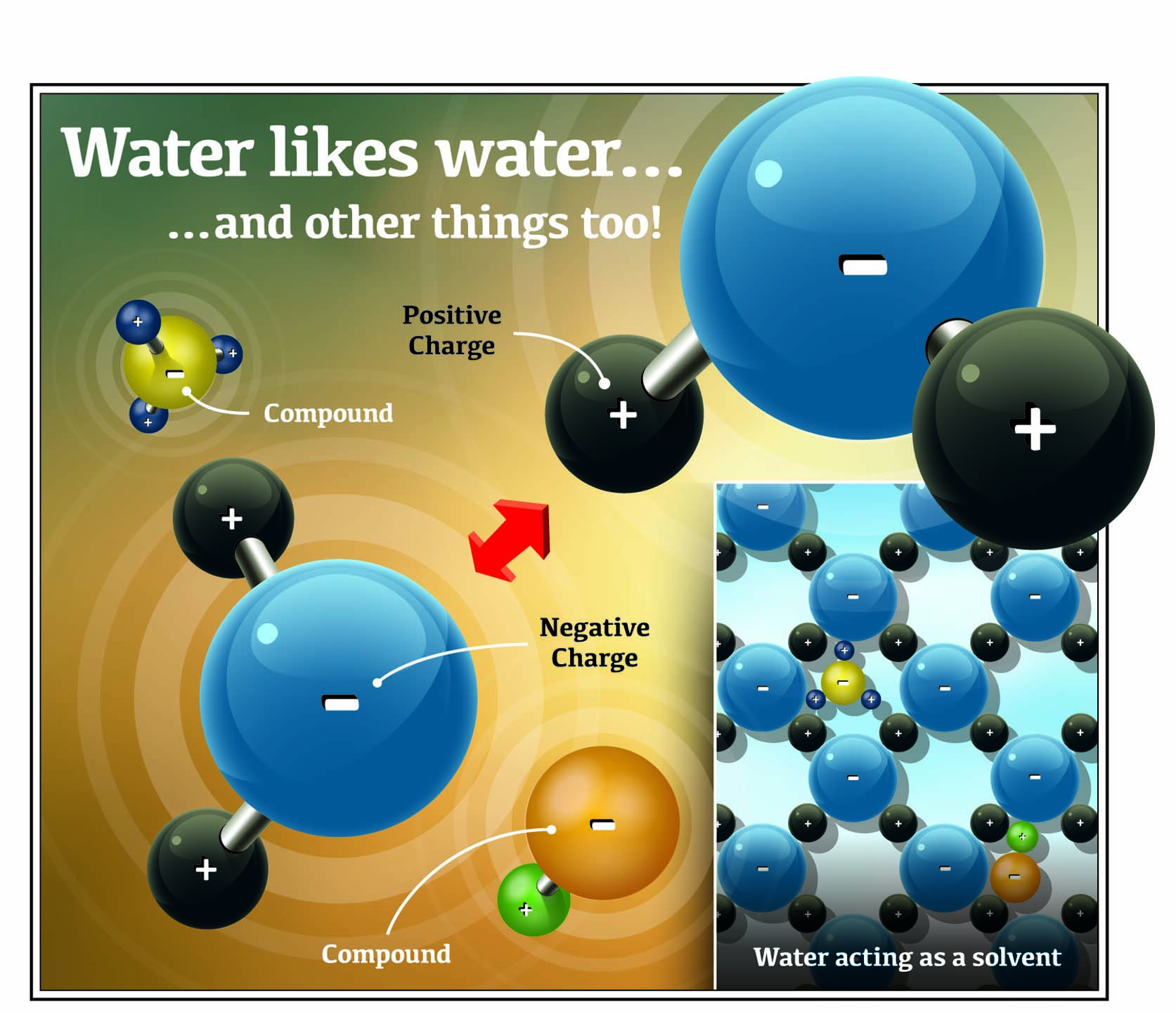
We all know the molecular formula for water: H2O. The formula creates the illusion of a simple molecule, but in fact, water is anything but simple. Water is a biologically active molecule with chemical and physical properties that make it an amazingly complex compound.
Here are eight properties of water that you should know before applying pesticides.
1. Water Is Not Pure
We assume water from a spigot is pure because it looks clean and tastes good. But that water typically contains varying amounts of dissolved minerals such as calcium and magnesium — they give water its taste. We further assume that if water is suitable for drinking, then it’s adequate for mixing pesticides. This is not necessarily true.
Calcium, magnesium, and iron dissolve in water as it moves through the soil profile (in the case of water from wells) or moves across the surface (in the case of surface water). So most water isn’t pure because it contains some amounts of these dissolved minerals. Water’s interaction with the environment determines whether it is hard or soft, acidic or alkaline. All of these factors can affect pesticide performance.
2. Water Is Not Simply the Pesticide Carrier
Water is more than a spectator when it comes to diluting and delivering pesticides; water’s chemistry makes it an active participant. Water can influence pesticide performance any time from the tank mix to when it reaches the leaf surface.
3. Water Quality Can Vary Dramatically Within a Few Miles
Most people know water is significantly different across regions of the United States. The source of the water, limestone deposits, well depth, recharge area, and annual rainfall amounts all influence water quality.
But what is often overlooked is that water from two sources that are close to one another can also differ significantly in chemical composition. The properties of surface water may be completely different from the groundwater in the same location, even the same farm. These differences are not obvious to the naked eye and can affect pesticide performance.
4. Water Is a Polar Compound
Water (H2O) consists of two hydrogen atoms (H) and one oxygen atom (O). The oxygen atom in water has the effect of drawing the electrons it shares with hydrogen atoms. As a result, the electrons spend relatively more time in close proximity to oxygen.
Electrons are negatively charged, so they make the oxygen atom somewhat more negative. At the same time, each hydrogen atom becomes somewhat more positive. This unequal charge makes water a polar compound — each side of the molecule is like the two sides of a magnet.
Because of these polar properties, water is extremely attractive to other polar compounds, including itself. This property allows water to act as both a solvent and carrier for pesticides. This attraction allows water to form a cohesive lattice. Very often, this association is stronger between water molecules than the surface it contacts.
Water has strong cohesive properties (interconnectness) rather than adhesive properties (like a paste). Think about water droplets running down a windshield. As a droplet moves down and hits another, both droplets merge into a single drop. This interaction is stronger than the spreading of the same droplet across the windshield and is responsible for the effect we call beading.
There are even times when water can move up or against gravity. For example, when the upper layer of soil begins to dry, capillary action can wick water up into the soil profile, which is due to water’s affinity for itself. So, as water evaporates at the surface, the strength of association between water molecules draws more water from below the surface up to replace what was lost.
5. Water Is an Excellent Solvent
Water’s polarity allows some products to disperse and dissolve in the structure of water. Polar pesticides are attracted to water just like water is attracted to itself. When this attraction occurs between water and a polar organic solid, the solid material is said to go into solution.
Dissolving sugar or salt in water are common examples of water’s effectiveness as a solvent. The molecule of the herbicide glyphosate is polar, so it dissolves in water like table salt would. Because it bonds with water, glyphosate will dissolve and remain in solution without the need to further agitate the spray mixture. It is said to form a true solution in water.
Some pesticides are oil products that are insoluble in water. These products are significantly less polar than water molecules or completely nonpolar (that is, they are neutral or have no charge).
Instead, of dissolving in water, these products are dispersed in it and never form a stable mixture by themselves, even after thorough mixing. Without agitation, nonpolar pesticides will stratify into distinct layers on the top or bottom of a spray mixture, depending on their density relative to water. This is why manufacturers formulate some pesticides with emulsifiers to prevent separation.
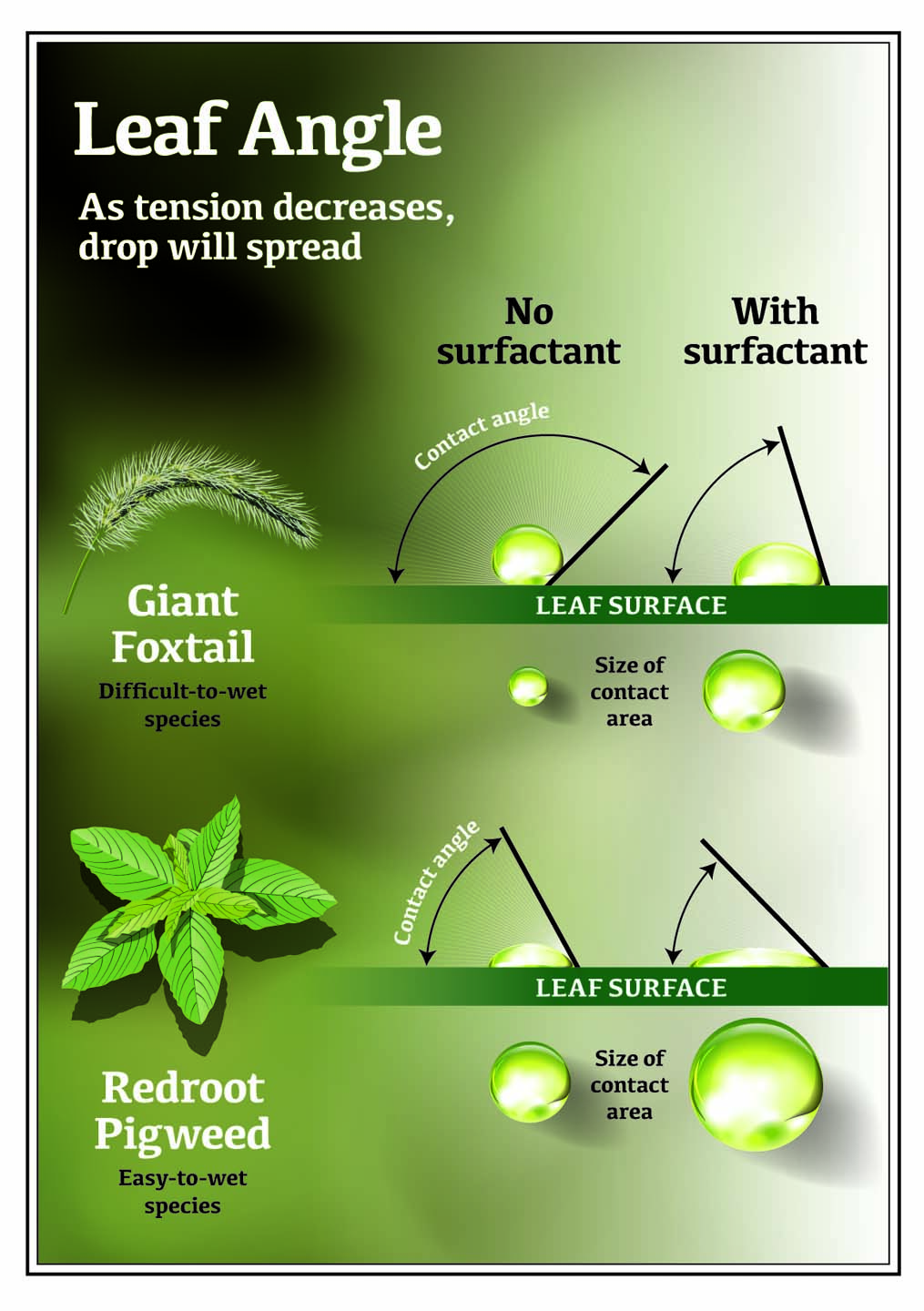
6. Water Has High Surface Tension
Have you ever noticed a water droplet sitting on a leaf is almost always spherical? That’s because the properties of water and air create surface tension, the force that allows a liquid to resist an external force (like air). The surfaces of plants also play a role.
As long as a droplet has high surface tension, the water droplet will remain upright and not spread over the leaf. Such droplets have a high contact angle, which means the droplet covers less surface area. A lower contact angle means the droplet covers more surface area.
For pesticide applications, when a water droplet doesn’t spread and has a high contact angle, it is generally perceived as a poor application. Too much spreading and too low a contact angle may also present problems. Finding the right balance of spread and contact angle are important for the specific pesticide.
7. Water Is a Volatile Molecule
Volatility is the tendency of a substance to vaporize. Water is a volatile ingredient in spray applications, especially when the relative humidity is low.
Volatility affects the spray droplet in two ways. First, the spray can evaporate before it contacts the plant. Second, the droplet on the leaf can evaporate before the pesticide has a chance to enter into the plant. Neither is desirable.
8. Water Has No Inherent Buffering Capability
A solution that resists changes in its pH is a called a buffer. For example, a pH 5 solution is acidic. Let’s say you add a pesticide into the mix that has a mildly alkaline pH of 7.5. If the solution’s pH remains at or near 5, then the original solution is considered a buffer because it can resist the shift in its pH.
On the other hand, water is not a buffer and will take on the characteristics (pH, buffering) of the products placed in it. As a solvent without buffering capabilities, water’s pH can readily shift up or down, even if you add a small amount of an acidic or alkaline material.
The degree of pH change in a water-based pesticide spray mixture will depend on how much material you add and the material’s actual pH and solubility. Manufacturers often formulate a given solution’s pH to optimize performance. For example, Monsanto optimizes the formulation of Roundup® so the solution has a pH of 4.5-5.5.
Each pesticide has unique chemical and physical properties that can influence how it interacts with the leaf surface and carrier (water or otherwise). Many pesticides respond to differences in pH, dissolved minerals, and water temperatures. That’s why it’s important to understand the pesticide molecule to maximize its potential.
Here are four things to remember about the properties of pesticide molecules.
1. Pesticide Solubility and Degradation Rates Vary
A spray solution’s pH plays an important role. The solution’s pH determines how much of the pesticide will go into solution (solubility) and how quickly the pesticide will degrade or break down once dissolved.
Each pesticide molecule has a pH range that is optimal for its solubility and chemical stability in water. A pesticide’s half-life describes how long it takes for half of a pesticide to be destroyed or degraded. Changes in pH can make a pesticide’s half-life range from minutes to months.
For example, some carbamate insecticides have a half-life of 10 minutes in alkaline solutions. And sufonylurea herbicides are far more soluble in solutions that are more alkaline.
2. Opposites Attract
Negatively-charged pesticides bind to positively-charged molecules in water. For example, the active ingredient in formulated glyphosate has a negative charge, so it associates with minerals in soil or dissolved in water that have a positive charge. The associations can be strong or weak. The stronger the association, the more likely it is that pesticide performance will be poor.
When pesticides bind to certain minerals (particularly iron, calcium, and magnesium) plants do not absorb them as well. This is because the pesticide, after binding to the minerals, may have become too large to penetrate the plant.
Frequently, the combined molecule (pesticide and mineral) has a reduced solubility, which may result in solid particles precipitating out of the spray mixture. When this happens, less of the active form of the pesticide is available. This process is called hard water antagonism — the pesticide is still there, but it is in a form not readily available to the plant.
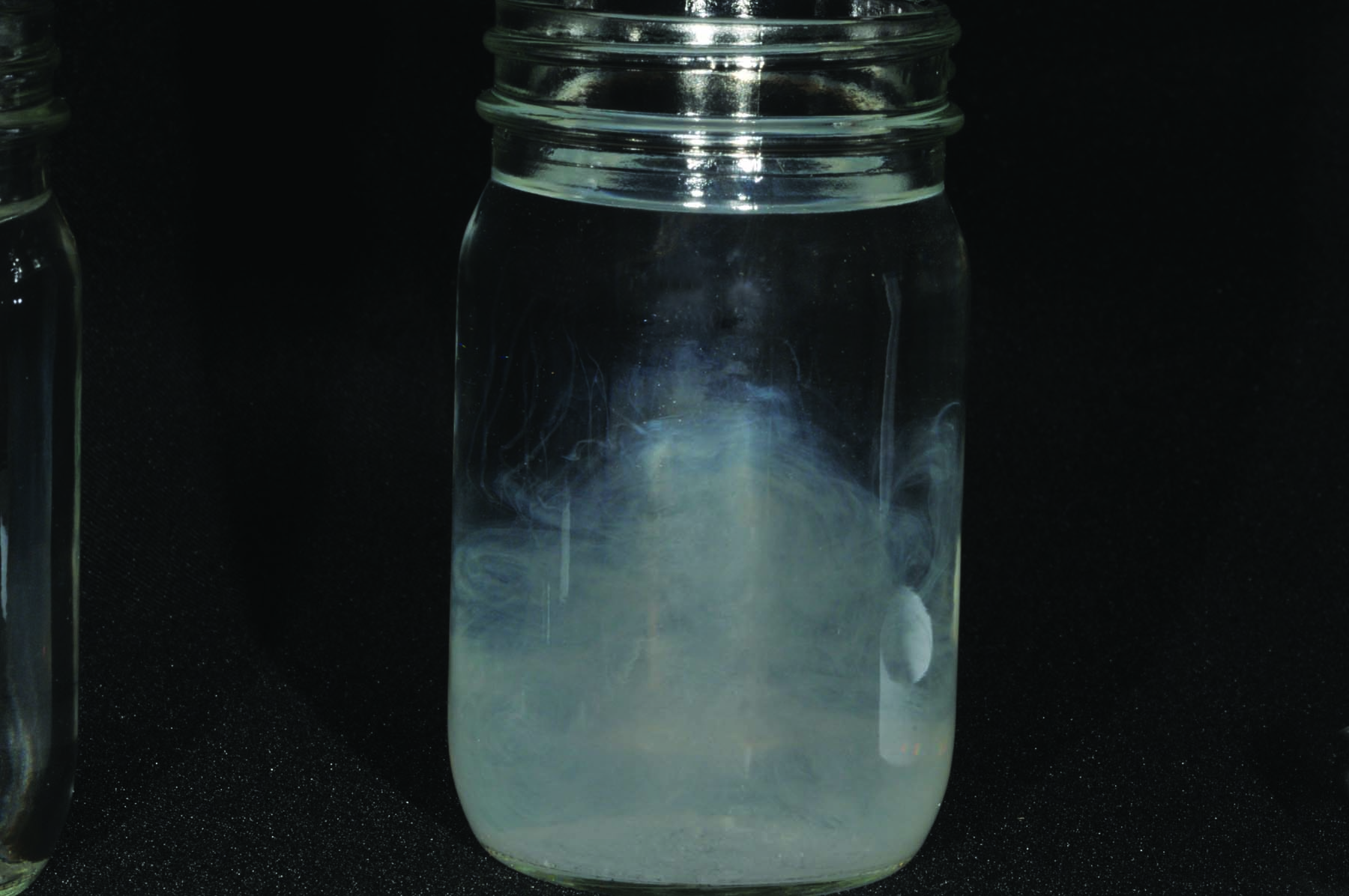
The more minerals the water contains (the harder it is), the more certain pesticides will be “deactivated” by binding to minerals like calcium, magnesium, and iron.

In these photos, the bottles on the top contain distilled water with no minerals (zero hardness) while the bottles on the bottom contain minerals. The bottles on the bottom turn cloudy after adding a product that mimics glyphosate while the bottles on the top remain clear. The product is in solution in the bottles on the top; calcium has bound to the product in the bottles on the bottom.
3. Pesticides Enter Plants Through Passive Diffusion
Many contact insecticides and fungicides work at or near the leaf surface and have limited movement through plant tissue. All herbicides and locally systemic insecticides and fungicides, must enter a leaf to be effective.
Systemic herbicides must, after entering living plant cells, move relatively long distances in the plant to reach the growing points in the roots and shoots. Successful pesticide movement in the plant depends on many forces, including pH, solubility, and whether it moves in the phloem or xylem.
Successful absorption of systemic pesticides depends on:
• Getting the right size of spray droplets
• Delivering droplets uniformly across leaf surfaces
• Keeping droplets on leaves long enough
Systemic pesticides enter plants through a process called passive diffusion, which means that the pesticide moves from a region of higher concentration (typically the spray deposit) to a region of lower concentration (typically the inside of a plant).
You can see passive diffusion at work if you place a drop of dye in a glass of water. It only takes a few minutes for the dye to disperse from the drop throughout the glass — it is moving from a higher to lower concentration. You’ve also experienced passivediffusion if you’ve ever caught the aroma of coffee or freshly baked bread drifting from the kitchen to another room.
Gradually, the pesticide molecules that have been dissolved in the spray solution migrate into the plant. The time it takes for an effective amount of the pesticide to move into the plant can vary greatly depending on the:
• Pesticide and its properties
• Plant species
• Plant’s leaf structures (see Leaf Surfaces Affect Pesticide Performance, page 17)
• Thickness of wax layers
4. Environmental Factors Play a Role
Environmental conditions at the time of a spray application affect pesticide performance. The most important effect of environmental conditions is the rate of evaporation.
When humidity is low, water droplets can quickly evaporate and leave behind a dry pesticide residue on the leaf surface.In this dry, solid phase, pesticides with polar characteristics (most commonly
water-soluble pesticides and their salts) are either unable to penetrate the leaf surface or they penetrate slowly. Pesticides with nonpolar characteristics may still penetrate the leaf, but movement is much slower than when the deposit is still in liquid form.
Leaf Surfaces Affect Pesticide Performance
Leaf surfaces are one of the more complex biological structures known to scientists. Plant surfaces harvest light, breathe, exchange water, have natural defenses, resist physical forces, and more. A leaf surface’s composition (whether it is waxy, hairy, and so on) plays an important role in how a pesticide spray droplet spreads over the leaf surface and penetrates it.
The outermost part of a leaf is called the cuticle, which is a protective film. The composition of any given plant’s cuticle creates significant considerations for the spray droplet, including:
• How well spray droplets cover the leaf
• How well spray droplets are retained on the leaf surface
• How spray droplets behave
• How pesticides move into plants from retained and drying spray droplets
Of course, these considerations are closely linked to the five important physical and chemical properties of the way spray droplets interact with leaves described on the following pages.
1. Spray Droplets Must Contact Leaves
It is more difficult to get spray droplets to hit and remain on narrow, upright blades of grass than it is on wide, horizontal broadleaf plants.
But it is critical that the leaves of any target plant are able to retain as many pesticide spray droplets as possible.
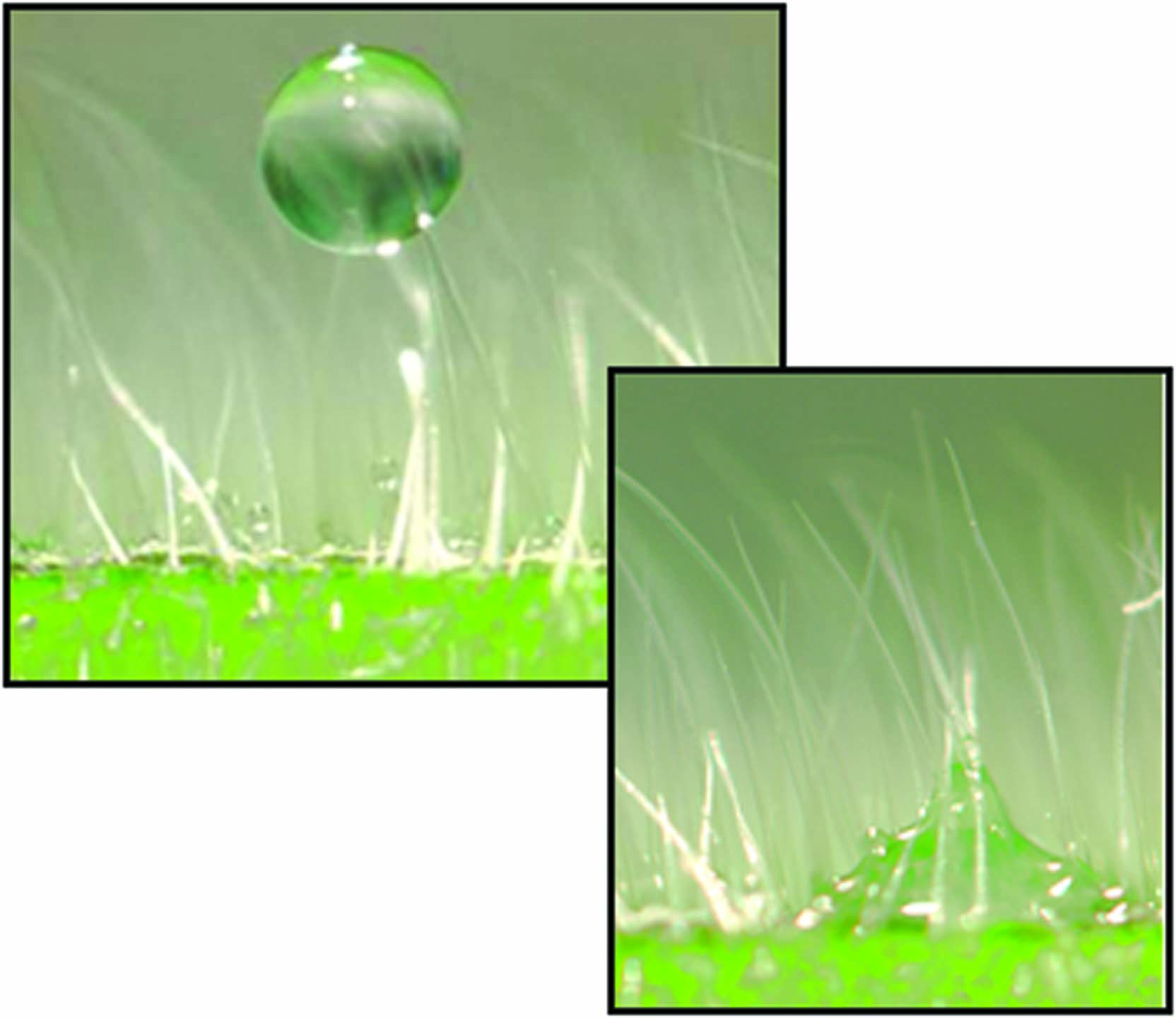
2. Plant Hairs and Other Structures Keep Droplets Off Surfaces
Pesticides are most effective when the spray droplets directly contact the leaf surface. Plant hairs (trichomes) can suspend droplets above the leaf surface until they evaporate.
Trichomes that point vertically up from the leaf surface are typically coated with wax. Research shows that trichomes are not a major entryway into the leaf (although some may absorb slight amounts). The bottom line: unless a spray droplet properly contacts the leaf surface, pesticide uptake will be minimal.
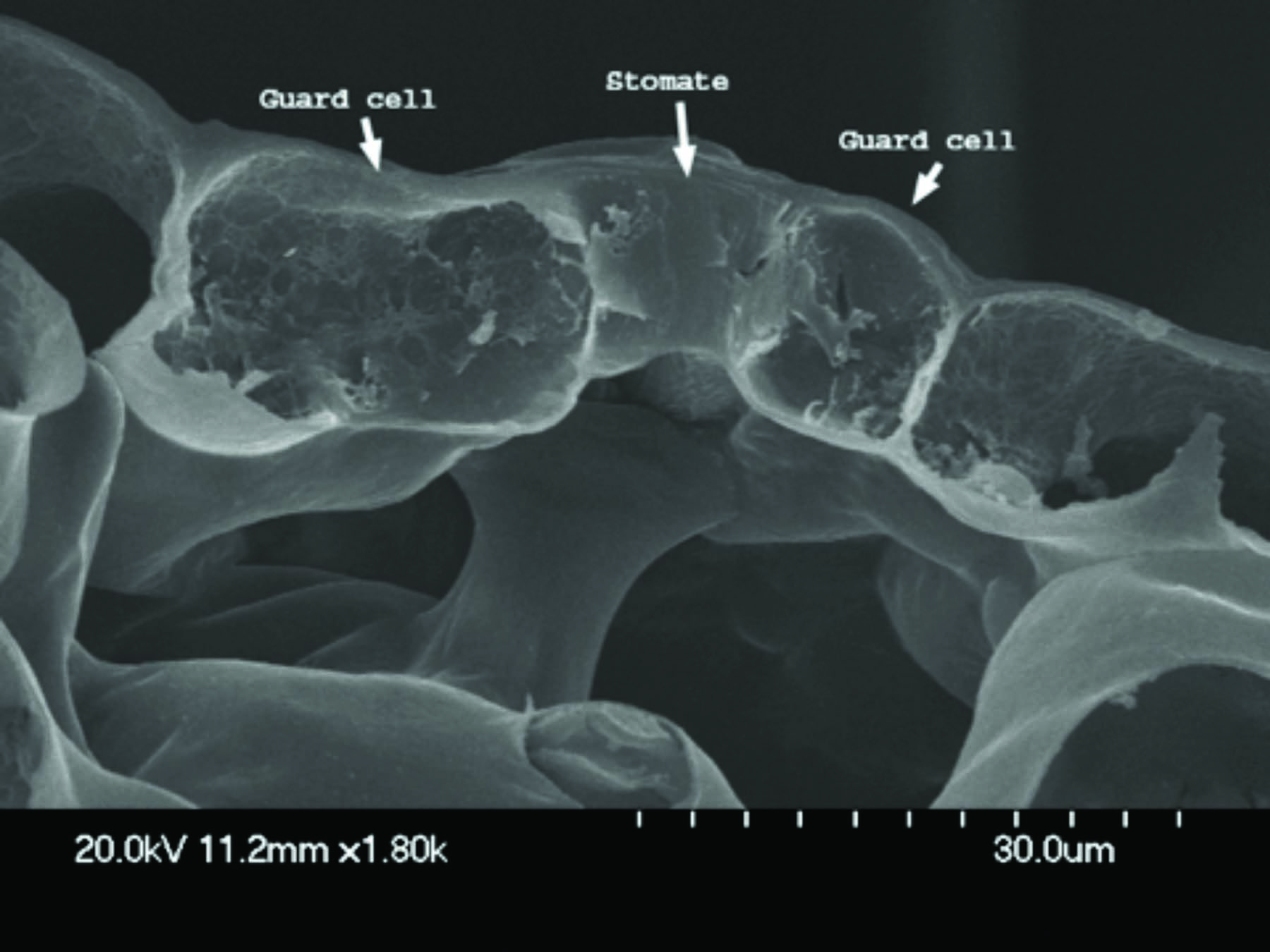 Research indicates that certain pesticides may enter plants through pores called stomates, which are located on the upper and lower leaf surfaces. Stomates open and close to release gases from the leaf. There is no positive correlation between the number of stomates and the amount of pesticide a leaf absorbs. This indicates that stomates are not important routes of pesticide uptake.
Research indicates that certain pesticides may enter plants through pores called stomates, which are located on the upper and lower leaf surfaces. Stomates open and close to release gases from the leaf. There is no positive correlation between the number of stomates and the amount of pesticide a leaf absorbs. This indicates that stomates are not important routes of pesticide uptake. 3. Leaves Must Retain Spray Droplets
Even when a pesticide spray droplet lands on a leaf, the droplet has to remain on the foliage to be effective. When a spray droplet leaves the spray nozzle it has speed and mass, so it has momentum.
At the moment of impact, the leaf surface acts like a shock absorber, bending and stretching as the spray droplet contacts it. After contacting the leaf, the droplet can remain on the surface, shatter, bounce off, or roll off the edge. How that droplet responds has as much to do with its physical properties as it does with those of the leaf surface.
4. Waxes Repel Spray Droplets
The waxy cuticle on leaves can make it difficult for pesticides to enter plants (through passive diffusion) and for water to escape the plants. By itself, water can do little to carry a pesticide across the cuticle barrier and into the target.
Pesticide solubility and penetration can present one of the most significant barriers to pesticide uptake because leaf surfaces can repel water (they are hydrophobic). At the leaf surface, the waxes and other substances in the cuticle can create a nearly impenetrable barrier for water-based sprays. Polar water droplets and nonpolar waxes don’t interact well; one molecule repels the other. The result is similar to what you would see with water on a waxed car.
Less polar pesticides are more soluble in the waxy portions of the cuticle, which more readily allow dissolved pesticides to move across this barrier layer.
Just how quickly a spray solution can penetrate the cuticle depends on how much of the pesticide is in a dissolved state — solid or crystallized pesticides do not readily enter the leaf (see Water Is a Polar Compound, page 12). Spray adjuvants can help improve pesticide uptake. With polar, water-soluble, organic, or inorganic pesticides the challenge is how to help the spray droplet deliver the dissolved pesticide across the impermeable leaf layer.
SEE DROPLETS ON LEAVES
The USDA-Agricultural Research Service has videos of spray droplets contacting leaf surfaces:
www.ars.usda.gov/Services/docs.htm?docid=19299


5. Plants Protect Themselves With Wax Layers
Plants that grow during extended periods of high temperatures, low humidity, and prolonged drought can produce thicker coatings of surface wax. This process, known as hardening off, helps prevent plants from drying out. Hardening off makes it even more challenging for pesticides in a water-carrying solution to stick to, spread over, and penetrate a plant’s surface.
Plants may also alter the composition, thickness, and molecular arrangement of the wax in response to age, environment, and even leaf position on the plant. When foliar-applied herbicide applications fail to control the target during droughts, a thicker layer of wax on the plant’s cuticle (which limits absorption) is commonly the culprit.
FUNGICIDES REQUIRE GOOD COVERAGE
Some fungicides have limited mobility — they don’t spread across external plant surfaces well or translocate into plant tissue. Many common fungicides may penetrate leaf tissue and move only to the bottom surface of the leaf, or into the leaf tip.
Because of this, successful fungicide applications rely on good spray coverage. Fungicide product labels often recommend using a spray adjuvant to enhance the spreading, penetration, and mobility of the pesticide.
Considerations for Mixing Pesticides
There are many things to think about when you mix pesticides. Here are two important factors.
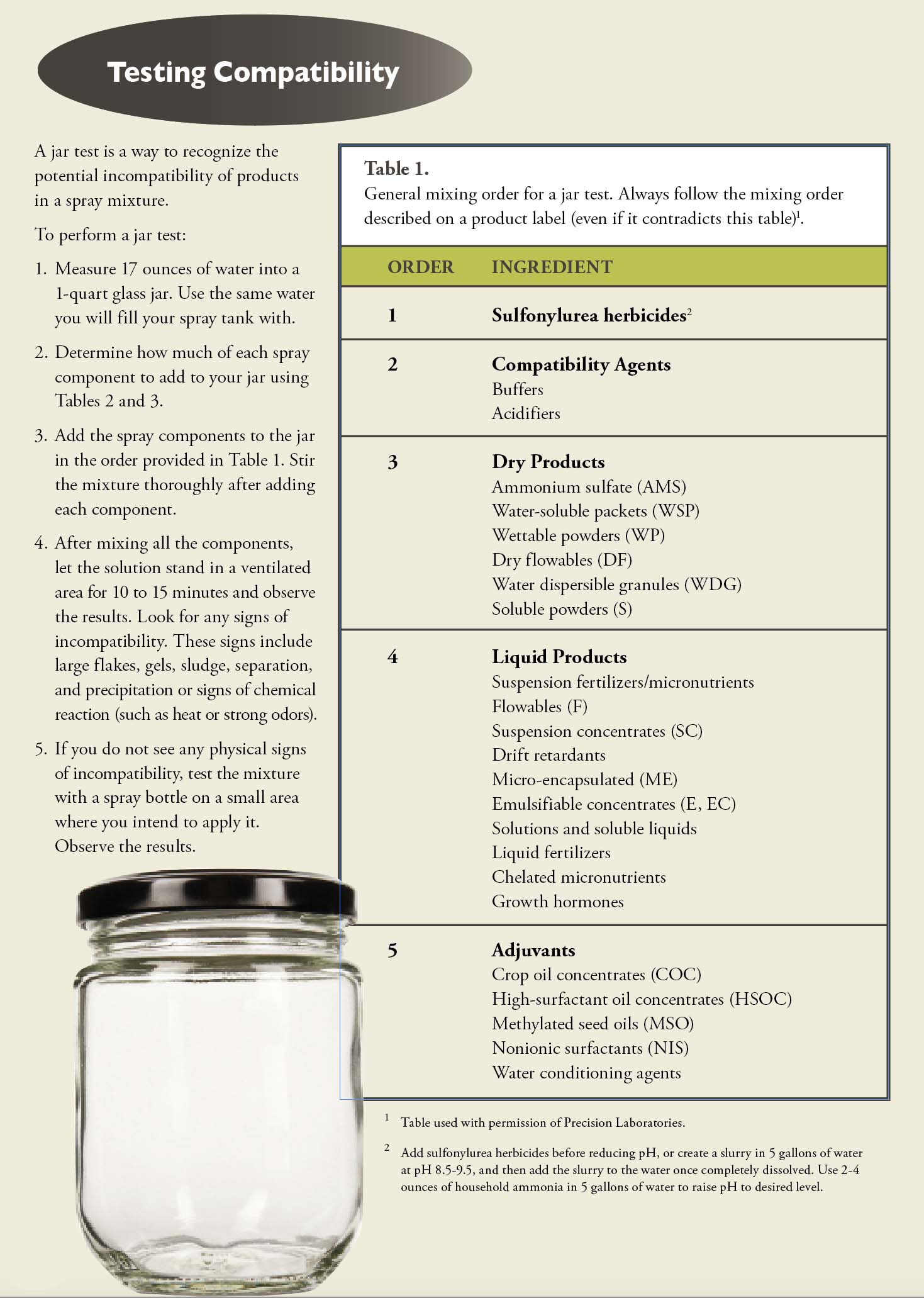
1. Mixing Order Affects Solubility and Compatibility
The order you add pesticides and other materials to the spray tank can influence how well the products work together. Mixed in the wrong order, the different products may clump together to form a paste or gel instead of remaining in solution or suspension. Once this happens, performance is almost certainly reduced (if the resulting mixture can be sprayed at all).
Pesticide labels often provide the preferred order that you should add products to the mix. If you add pesticides, fertilizers, and other tank adjuvants in the wrong order, it can create physical incompatibilities that may result in adverse changes in pH, reduced solubility, product separation, clogged nozzles, and reduced performance (see Testing Compatibility, page 36).
2. WaterTemperature Affects Solubility
Pesticides are more soluble in warm water than cold water. If you mix pesticides in cold water, add time to the mixing process to assure the products are fully dispersed, emulsified, or dissolved.
Pesticides that are sold as water dispersible granules (WG) disperse slower in cold water than in warm water. Allow an extra two or three minutes for mixing if you add WG products to cold water. You want to make sure the granules have fully disintegrated.


Visually inspect the spray mixture closely before adding other products, especially before tank mixing other oil-based formulations. If you add an oil-based product to a spray mixture before a granular formulation is fully dispersed in the water, you may encounter application problems. You may observe oil-coated clumps (agglomerates)collecting in the filters and nozzle screens of your application equipment.
Water-based formulations of pesticides, including suspension concentrates (SC), concentrated oili n water emulsions (EW), suspension/emulsion pre-mixtures (SE), and even some soluble liquid (SL) products tend to thicken when cold. When cold, these products also pump and pour more slowly and often require more time to fully mix in cold water simply because they have a higher viscosity than when they are warm.
When these products are warm and you add them to cold water, they generally dissolve quite well; however, it may still help to slow the rate you add the product to the spray mixture and slightly increase the agitation.
Oil-based products — including emulsifiable concentrates (EC) and oil dispersions (OD) — are the formulations most sensitive to water temperature. Very cold (or very warm) spray water tends to change the product’s emulsification behavior. Temperature extremes in water tend to lead to less stable emulsions that separate more rapidly when left unagitated. In worst cases, this can also lead to product oiling in the spray mixture — oily product residues form on spray tank surfaces.
Generally, it is best to apply oil-based products shortly after you mix them. If you don’t agitate such mixtures, it generally makes matters worse, and can contribute to tank-mix compatibility problems with other pesticides. In addition, you may need to clean out your tank more rigorously before using it again.
The temperature of the water you use in spray mixtures is an important variable for problem-free pesticide
applications. Use extra caution if you use very cold (< 50°F) or very warm water (> 80°F). If you must use cold or warm water, be prepared to add some mixing time.
Understanding Application Equipment
Spray application technology is intricate and aims to produce a spray droplet at a desired size, and then deliver that droplet intact within a specific size range to a target site. A spray droplet’s size and how it deposits on a plant depends on your equipment and sprayer settings, external environmental factors during (and up to 24 hours after) application, and the chemical properties of the spray mixture itself.
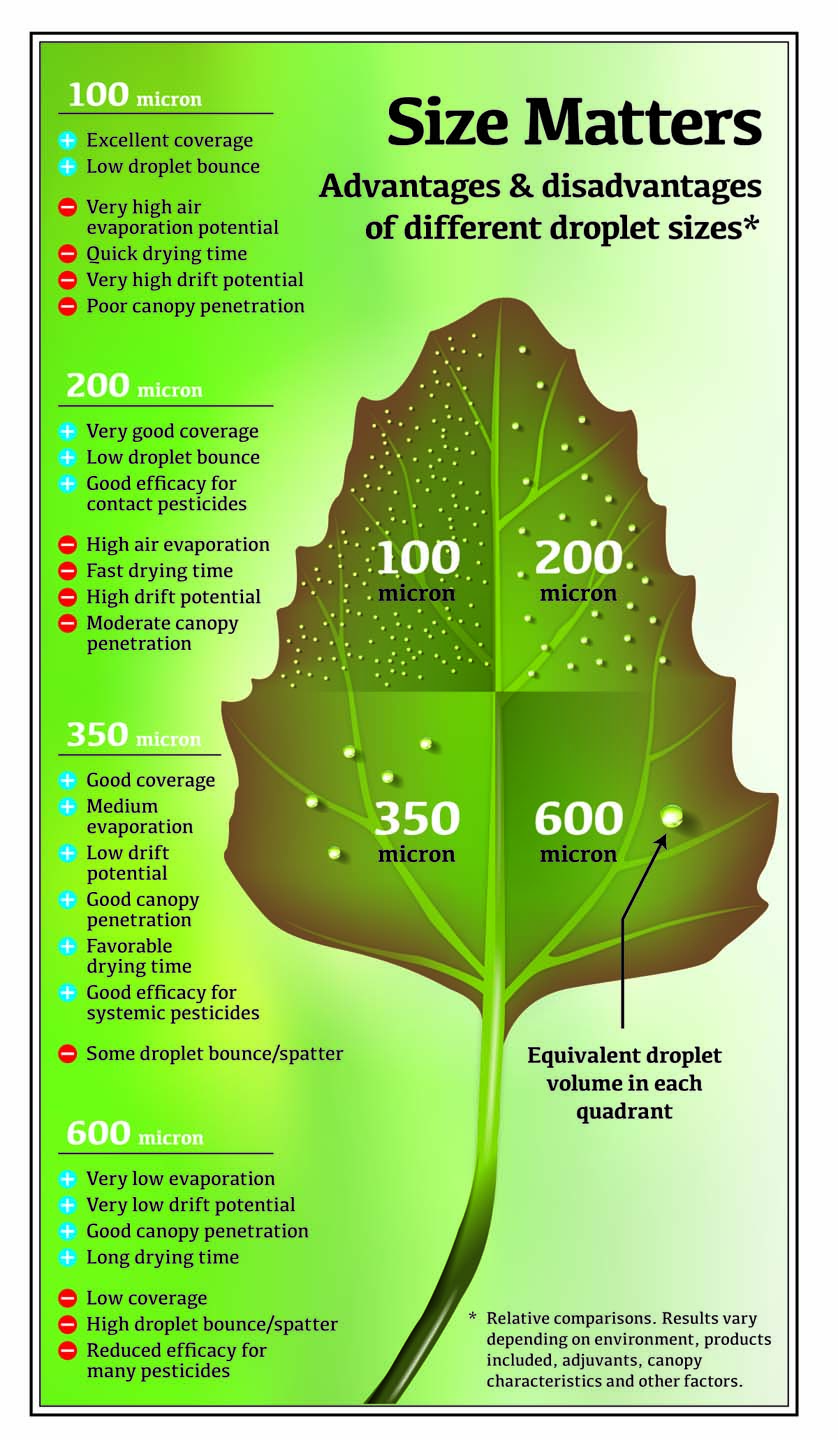
If the size spectrum of the droplets is incorrect (the drops are too large or too small), you waste time, product, and money. Nozzles that emit droplets that are 150 microns or smaller can potentially improve coverage because they produce eight times as many droplets as a nozzle that emits 300-micron droplets. However, such small droplets almost always compromise coverage because fine droplets also increase the potential for off-target drift and evaporation loss.
Conversely, nozzles that create large droplets also create fewer droplets that have a greater potential for bouncing, shattering, and running off the target. The bottom line is that droplets that are too small or too large can lower application efficiency. For every product and pest, you want your application equipment to generate the optimal spray droplet size.
Here are three things to consider about spray droplet size and your application equipment.
1. Many Factors Influence Spray Droplet Size
When a nozzle discharges a spray mixture, it first forms a liquid film. After encountering air, the film breaks into segments, which eventually become a range of droplet sizes (the droplet spectrum).
Spray droplets are measured in microns, which are equal to 1/25,000 of an inch. A human hair’s diameter is 100 microns; a toothbrush bristle is 300 microns.
Droplet size, distance to target, and wind speeds are important factors in predicting off-site pesticide movement.

The smaller the droplet, the greater the potential that the wind can carry the droplet from its release point.
Small droplets have a low terminal velocity: their low kinetic energy causes them to decrease speed faster, float longer, and evaporate more quickly than larger droplets.
Large droplets have a high terminal velocity, so their relatively greater mass and higher speed make it easier to manage their trajectory. And because larger droplets contain more volume, it takes longer for them to evaporate. They still have the undesirable aspects of bounce and runoff, which reduce their retention on leaf surfaces and can cause noticeable reductions in pesticide efficacy.
Factors that influence spray droplet size include nozzle design, spray pressure, spray angle, travel speed, orifice size, pesticide and adjuvant characteristics, and distances from nozzle to target. Remember that the spray droplets your nozzles will produce during application are not uniform in size. Therefore, the spray properties are defined by a number of measurements. The most frequent measurement is commonly referred to as spray quality, which is a standardized way of characterizing agricultural sprays.
2. Success Depends on a Certain Number of Droplets
To be effective, a certain amount of a pesticide must remain on a leaf surface. The deposit pattern depends on the droplet size, spray direction, droplet velocity at the point of impact, physical properties of the spray mixture, and characteristics of the leaf surface (angle, wax, trichomes).
For pesticides to work properly, the target area needs retain a minimum amount of spray mixture at sufficient concentration for plant uptake. Generally, this is accomplished using droplets that are between 150 to 300 microns to balance the potential for drift with the potential for good coverage.
When droplets are small, more of them can be deposited on a leaf (coverage) and the untreated area between droplets can be decreased (droplet density). Small droplets are also likely to remain on the leaf surface and are less likely to bounce, shatter, or run off. The question is typically what proportion of these droplets will actually reach the target.
The problem with larger spray droplets is that they hit the plant at higher speeds and with greater momentum, which means larger droplets have a greater chance of landing on the ground. And if the leaf retains fewer droplets, then the pesticide’s performance can be significantly reduced.
Of course, it is more likely that larger droplets will not drift and at least reach the target site. Effectively using larger droplets is critical to maintaining herbicide efficacy while minimizing any potential off-target spray drift that can result with smaller droplets.
3. The Environment Slows Droplets
Pressure in the spray boom creates the energy to create and expel spray droplets. When spray droplets hit the leaf, most are traveling between 9 to 16 feet per second (6 to 11 miles per hour). As water droplets exit the nozzles, uniform droplet speeds (velocity) depend on nozzle design, orifice size, and pressure.
Environmental conditions can affect droplet speed, especially when encountering warm temperatures or cross winds. Smaller droplets fall slower and evaporate faster than larger droplets.
Adjuvants — Overcoming Water-induced Barriers
To be effective, your equipment needs to apply a uniform pattern across the treated area. The technology and science of pesticide application equipment focuses on forcing the spray solution out the nozzle and forming spray droplets that will reach the plant with enough velocity. Once the droplets are sprayed, the mechanical portion of the application is complete.
In other words, once the spray leaves the nozzle, you can no longer control its fate. Each droplet can be viewed as an isolated snapshot of what was present in the tank. Nothing can be added and the only portions that can be removed are those that are volatile enough to evaporate as the droplets travel through the air or after they have hit the target surface.
By this time, it should be obvious that the time to consider and address such needs comes before spraying. One such consideration is incorporating one or more adjuvants in the spray mixture to predetermine how the spray behaves once it’s left the nozzle.
There are many things in a pesticide spray solution’s chemistry that could make it difficult to effectively apply.
To overcome some of these problems, some spray solutions can benefit from adding adjuvants. An adjuvant is a product that you add to a spray solution to improve the efficacy of a pesticide, consistency of the solution, and application safety (such as reducing drift).
An adjuvant can overcome issues with the:
• Barriers posed by the properties and quality of the water in the solution
• Structure and composition of target plant surfaces
• Application equipment and conditions
• Environment in which spray application is made
Adjuvants can improve pesticide performance by modifying the spray pattern, droplet, deposit properties (spray quality), and rate of movement of the pesticide into the plant (uptake and penetration). However, adjuvants are not magic bullets; they can only do so much.
Adjuvants can also improve the handling characteristics of a spray solution by:
• Keeping a pesticide from binding to minerals suspended in water
• Adjusting the water pH so a pesticide is less likely to break down
• Manipulating droplet size to reduce on-target and off-site movement of pesticides
• Improving the odds that a spray droplet will stay on the leaf by reducing factors that cause droplets to bounce and roll off leaves
• Modifying or reducing surface tension to enhance the ability of a droplet to be retained on or spread across the leaf surface
• Minimizing spray droplet evaporation
• Preventing spray deposit from being washed off the leaf surface
• Protecting the droplet from degrading in sunlight
• Improving a pesticide’s absorption and uptake by the plant

Adjuvant Classification and Nomenclature
You can’t choose the proper adjuvant until you understand the full range of performance needs of each spray operation.
Suppliers, distributors, and manufacturers have developed standardized terms to classify and describe different adjuvants, which are recognized by the American Society for Testing and Materials (ASTM International or ASTM). Fortunately, the type names are descriptive enough that you can deduce what the adjuvant will do.
The following pages describe 13 types of adjuvants for pesticide applications.
1. Water Conditioning Agents
Water conditioning agents (or water conditioners) are adjuvants that bond with dissolved hard water ions such as magnesium and calcium. They provide “sacrificial” molecules to keep pesticide molecules from bonding to the hard water ions. In chemistry, this process is called chelation. Tying up these minerals allows the target plant to more readily absorb the pesticide.
The most common water conditioning agents are ammonium sulfate (AMS), dipotassium phosphate, or a low-dose alternative to AMS.
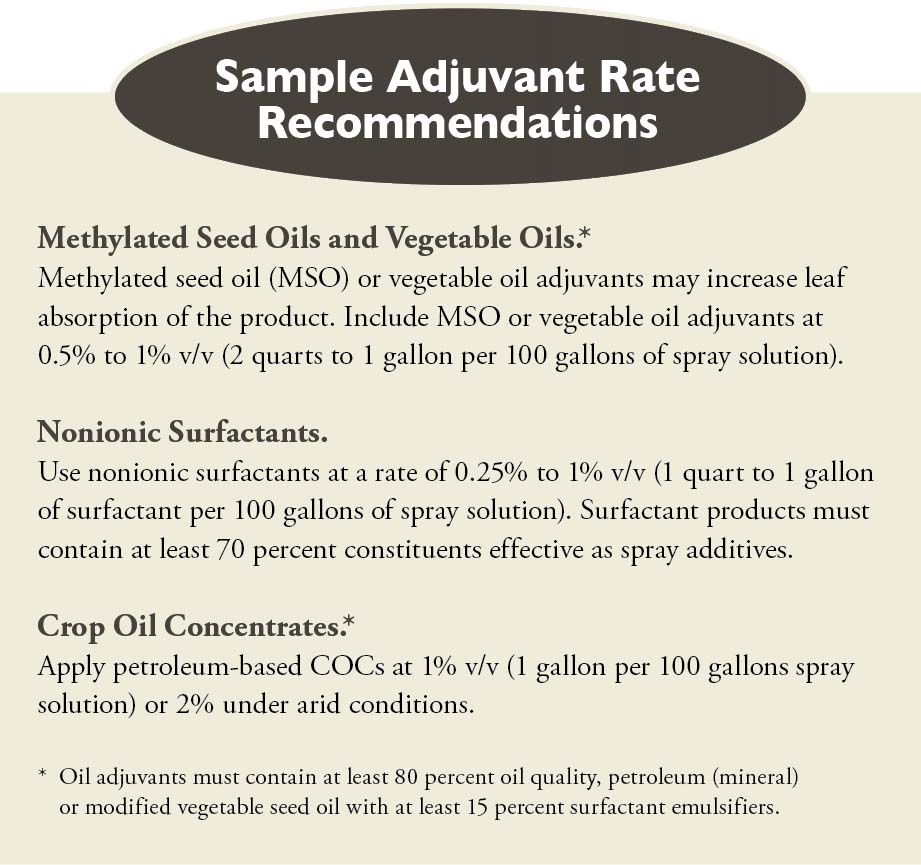
You can use dry “spray grade” AMS at a rate of 8.5 to 17 pounds per 100 gallons of water to counter the effects hard water cations such as calcium, iron, and magnesium. Liquid AMS solutions at rates of 2.5 to 5 gallons per 100 gallons of water will provide similar effects.
As the name suggests low-dose alternatives for AMS require less product — about 2 to 6 pints per 100 gallons of water. The chemistry of these products allows them to sequester hard water cations, but product performance across brands is highly variable and the low dose rate is intended to be sufficient for average water sources, so they may be ineffective for water that is significantly harder than average.
Liquid AMS products generally require a volume concentration of 2.5 to 5.0 percent on a volume to volume basis (or v/v — 5 gallons of product per 100 gallons of water is a 5% v/v). An alternative is to add a liquid or dissolved water conditioning agent that contains a chemical that will bind with calcium and magnesium. It must still be at least as effective as ammonium sulfate for conditioning hard water, but capable of being used at lower rates (such as 1 percent depending on water hardness).
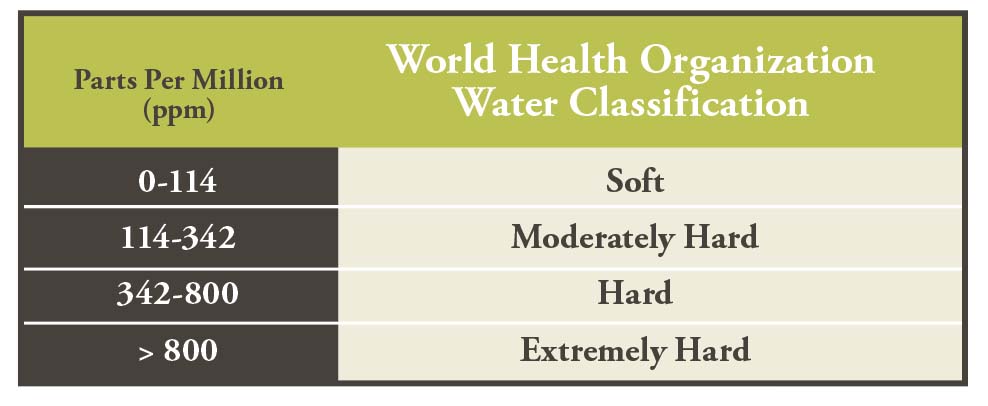
HOW MUCH AMS DO YOU NEED?
To know how much ammonium sulfate (AMS) you need to add to water in order to overcome
antagonistic ions first requires a water test. Once you know how much potassium (K+), sodium (Na+),
calcium (Ca+), magnesium (Mg+), and Iron (Fe+) are in the water, you can calculate the dose this way:
(0.002 x ppm K+) + (0.005 x ppm Na+) +(0.009 x ppm Ca+) + (0.014 x ppm Mg+) + (0.042 x ppm Fe+) =
pounds of AMS to add per 100 gallons of water =
Let’s say a water test of a farm well indicated that the water had K at 2.37 ppm;
Na at 30.9 ppm; Ca at 55; Mg at 26.1; and Fe at 0.07. So:
(0.002 x 2.37) + (0.005 x 30.9) +(0.009 x 55) + (0.014 x 26.1) + (0.042 x 0.07) =
1 pound of AMS to add per 100 gallons of water
Source: North Dakota State University
2. Surfactants (Wetter-spreaders)
Surfactants (also called wetter-spreaders) are adjuvants that lower the surface tension of spray droplets (measured in dynes/cm). This allows gravity to collapse and flatten the spherical spray droplet to the leaf surface, which in turn allows droplets to spread over a larger area. Surfactants can also reduce the amount of bounce a droplet will experience when it hits a leaf, which allows for greater success at depositing.
Surfactants primarily work at the interfaces between the spray droplet, leaf surface, and surrounding air. However, they also can influence interfaces both inside and outside the leaf or the intended target pest.
The surfactant adjuvants most commonly tank-mixed with pesticides are called nonionic surfactants (NIS). These surfactants are often made with materials that have an affinity for waxy plant surfaces.
Organosilicones are a type of NIS that are commonly used as tank-mix adjuvants with pesticides. Organosilicones are often called super-spreaders or super-wetters because they decrease surface tension so much. They also may have penetrant properties. Organosilicones greatly increase droplet spreading and decrease droplet sizes, which may be more desirable for some active ingredients and application conditions and less desirable for others.
Surfactants are some of the most commonly recommended adjuvants on pesticide labels. This is especially true for water-soluble and systemic herbicides. An NIS primarily spreads droplets and can, at times, enhance the uptake of certain active ingredients. However, NIS is typically less aggressive in penetrating the leaf than adjuvants that contain oil.
In applications when an NIS improves penetration, the more effective approach is to match the pesticide’s polarity with a surfactant of similar polarity. When you closely match a pesticide and adjuvant, like dissolves like, and you will increase the rate of movement for specific pesticides into the leaf. Many formulated pesticides already contain surfactants that can do this very effectively.

3. Oil Concentrates
Oil concentrates are adjuvants that help pesticides overcome leaf barriers to penetrate leaves better. They work because oily components soften or disrupt the waxes on leaf surfaces.
There are three general groups of oil concentrate adjuvants (based on their ingredients): crop oil concentrates, modified (methylated) seed oils, and high-surfactant oil concentrates.
Crop Oil Concentrates (COCs)
Crop oil concentrates (COCs) are adjuvants that contain petroleum-based (low-volatility) oils.
Modified (or Methylated) Seed Oils (MSOs)
Modified (or methylated) seed oils (MSOs) are adjuvants that are derived from plant seed oils. One example is biodiesel. In the United States, soybean oil is the most common MSO, although other oils can be used (such as canola, cotton, and sunflower). MSOs frequently contain 50 to 90 percent oil, enabling them to soften or break down waxes in the leaf cuticle.
The nonoil components of these adjuvants are usually surfactants. MSOs typically do not perform as well as surfactants at spreading when formulated at very high oil rates. For this reason manufacturers add more surfactants to these adjuvants to enhance wetting or spreading and to improve performance for water-soluble pesticides.
High-surfactant Oil
Concentrates (HSOC)
High-surfactant oil concentrates (HSOC) are adjuvants that were initially developed to improve control at lower use rates than COCs and MSOs. HSOCs also were developed to address the changing needs of tank mixes that contain glyphosate and are being applied to manage herbicide-resistant weeds.
Premium HSOC products may contain 20 to 40 percent NIS with 60 to 80 percent of the specific type of oil. These ingredients generally form more stable spray emulsions and may reduce use rates compared to comparable COC formulations that contain lower levels of surfactant.
Other crop oils may only provide enough emulsifying surfactant to allow you to mix and spray the oil. The oil in these tank mixes tends to separate to the top of the spray mixture fairly rapidly once you stop mixing and agitating them.
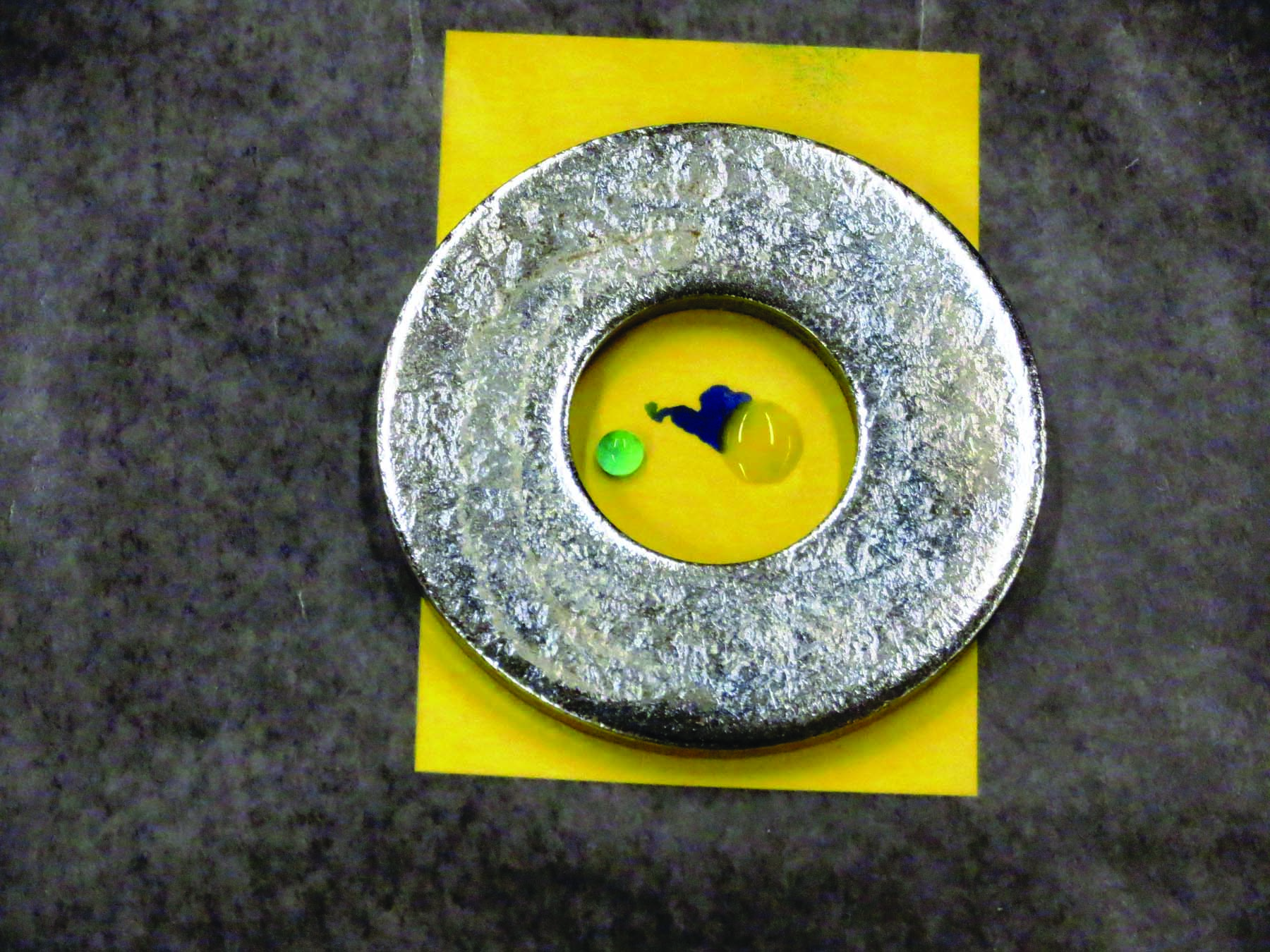
These photos demonstrate how an oil concentrate adjuvant helps a pesticide penetrate a leaf. (Top) A piece of paraffin was placed over water-sensitive paper that turns blue when it contacts water. (Bottom) The droplet on the left is water without an adjuvant. The droplet on the right contains an oil concentrate adjuvant. Notice the blue that indicates where the drop containing oil penetrated the paraffin.
4. Humectants (Evaporation Retardants)
Humectants are adjuvants that are synthetic or naturally derived and are designed to slow water evaporation in the air or on the leaf. Slowing the evaporation rate allows humectants to improve foliar uptake of some pesticides.
The longer the spray droplet stays in its liquid form, the more time certain pesticides will have to penetrate the leaf surface. This is particularly true of highly polar and water-soluble pesticides in situations when solubility is critical for the pesticide to be available to move into the plant. Once a polar pesticide dries on the surface (crystalizes), it no longer diffuses as rapidly into the leaf. For pesticide products that are not rapidly absorbed, prolonging the spray droplet’s drying time can be critical.
5. Ammonium Fertilizer Solutions
Ammonium fertilizer solutions are adjuvants that help pesticides that are sensitive to hard water. The fertilizer solutions that are considered adjuvants contain nitrogen (such as urea ammonium nitrate at both 28 and 32 percent UAN) and ammonium sulfate (AMS), which also is a water conditioning agent adjuvant.
When you mix certain herbicides with a foliar-applied ammonia nitrogen source, you can significantly increase the plant’s absorption and translocation of the pesticide. Many low-rate water conditioners do not contain enough ammonium ions to fill the adjuvant role that AMS can when a specific amount of ammonium is essential for performance.
6. Compatibility Agents
Compatibility agents are adjuvants that help incompatible products work together. They are most often used in complex tank mixtures that may include multiple crop protection products using multiple carrier systems. With so many possible product combinations, there will be occasions when products are incompatible.
Always check product labels for information about the compatibility of spray materials before adding them to a spray mixture. Compatibility refers to the physical and chemical interactions between two or more products in a spray mixture. Compatibility might be particularly problematic when the application has a high concentration of dissolved fertilizer, water conditioners, or micronutrients.
Compatibility agents are often recommended in concentrated liquid fertilizer mixtures to allow effective mixing and to avoid rapid oil phase separation of some formulation types. Other formulated compatibility agents may allow otherwise incompatible pesticide mixtures to be prepared and sprayed to maximize user convenience.
Even with a compatibility agent, it is critical to add each product to the spray tank or inductor in the proper order (see Table 1, page 36). For example, thicker products sometimes create mixing problems with other products.
Another example is water dispersible granules (WGs). Whenever possible, you should always add WGs first (that is, only to the water in the spray tank). If you do not allow the WG product to fully disperse in the tank before adding the next component, it can create sprayer problems because the formulation may coagulate or settle out in the mixture.


7. Defoamers/ Antifoamers
Defoamers/antifoamers are adjuvants that are used to manage or prevent air from being trapped in a spray mixture while preparing, mixing, or applying it. Antifoamer agents are most useful when they are in the tank mixture and prevent foam from forming. Defoamer agents are generally added to a mix to reduce foam after it has formed.
Some products inhibit foam from forming by reducing the surface tension of air bubbles, which allows them to revert to solution. Other products bridge and collapse the films that compose foam.
8. Scents (Masking Agents)
Scents (masking agents) are adjuvants that conceal the noxious odors of the pesticide or other solvents in the mix. These adjuvants are concentrated fragrances that may include specific odor control agents that reduce the volatility of more objectionable compounds.
Masking an odor is different from reducing a compound’s volatility.
Only affecting volatility relates to the pesticide’s potential for off-target drift.
9. Marking Agents
Marking agents (foam markers, spray pattern indicators) are adjuvants that incorporate dyes or foams that allow applicators see where they applied the spray mix. Applicators commonly use these adjuvants when spot spraying or working with invasive species.
10. Formulated Tank Cleaners
Formulated tank cleaners are adjuvants that, as the name suggests, clean the spray tank. There is no one-size-fits-all tank cleaner (always review product labels for recommendations).
Some tank cleaners are designed to degrade or deactivate the active ingredient of a particular product. Others are detergents that help remove oily film residues from equipment surfaces, hoses, and pipes. Still other tank cleaners may release and remove mineral deposits in the spray equipment that contain pesticide residues.
Adjuvants that contain ammonia and strong detergents remove traces of unwanted pesticides in the spray tank. Ammonia, with its high alkalinity, breaks down acidic pesticide residues in tanks and hoses. The detergents have an affinity for oil that may help to pull materials off the interior surfaces of spray equipment and that may have been adsorbed onto tank walls.
Ammonia and other materials also may degrade the remaining pesticide residues once removed from surfaces. Other more aggressive and less volatile materials may include sodium hydroxide or potassium hydroxide, which are very strong alkaline cleaners.
When you clean a tank or additional sprayer components (screens, strainers, lines), always make sure the cleaning solution remains in the tank long enough to effectively clean it, especially if you need to promote the degradation of pesticide residues. Always read and follow the tank cleaner label for specific directions about holding times.
If you want to remove hard water deposits and precipitated micronutrients from inside the equipment, include a chelating (or metal capture) agent.
High alkalinity may reduce the performance of chelating agents since mineral deposits are less soluble under alkaline conditions. In such cases, a mild acid cleaner may also be useful as long it is safe for the equipment to be cleaned.
It is best to decontomiate some difficult-to-remove or highly active pesticide residues (such as those from sulfonylurea herbicides) with an aggressive alkaline cleaner or a suitable oxidizing agent. Some of these cleaning agents (including chlorine and peroxide bleaches) may be available as consumer products. However, always be sure these products are compatible with your spray equipment before you use them.
Caution
Never mix ammonia with chlorine bleach because it can produce toxic chlorine gas.

11. Stickers
Stickers are adjuvants that help spray droplets better adhere to the target plant under windy or rainy conditions or from mechanical action. Stickers typically incorporate latexes (the material used in house paint to form a uniform, waterproof layer) or resins that help the dry spray deposit physically stick to the leaf surface.
Other products may spread with sticking (spreader-sticker). To do this, spreader-sticker products commonly contain surfactants mixed with sticky materials (such as fatty acids) to provide both adjuvant functions.
12. pH Adjusters (Acidifiers, Buffers)
The pH adjusters (also called acidifiers and buffers) are adjuvants that maintain or prolong a pesticide’s stability by modifying the spray solution’s final pH. Most pH adjusters are acidifiers that lower pH. Adjuvants that raise pH are less common.
Buffer and acidifier are often used interchangeably, but are quite different. Water has little or no buffering capacity (see Water Has No Inherent Buffering Capability, page 14), so a small amount of a pH adjuster can significantly alter the pH of the spray solution. An acidifier will lower the pH, but the pH of the resulting solution is still sensitive to any other compounds that may be added to it. To a certain point, a buffer can hold the spray solution’s pH at a given level even when you add products. This is helpful when the pesticide product is particularly sensitive to pH.
13. Drift Suppressing Agents (Deposition and Retention Aids)
Drift suppressing agents (deposition and retention aids) are adjuvants that help limit the drift of pesticide sprays off target. There are many different types of these products, so some function better in certain nozzles than others.
The performance of drift suppression agents depends on the spray mixture and application equipment. One type of agent will not fit all applications; each tank mixture may create different spray droplet distributions, so may require different products and use rates.
Some of these adjuvants thicken (build viscosity) in the solution to change the way spray droplets are formed. Some of the polymers that thicken the solution also help improve retention on the target by reducing the chance that droplets will bounce, shatter, or run off. However, polymers may also increase incompatibility issues. For example, they may not work well with certain drift reducing nozzles, producing a poor spray pattern or very large droplets that may affect pesticide performance.
Another type of drift suppressing agent works by reducing the percentage of smaller droplets — but it doesn’t necessarily make large droplets even bigger. These adjuvants are an oil-in-water emulsion or surfactant (such as lecithin-based products), or more traditional oil adjuvants (not all oils are effective drift suppressing agents).
The oil products work by producing many small and flexible oil-in-water emulsion droplets. When sprayed, these allow larger droplets to form without forming additional very large droplets. They may be more useful with certain nozzle designs and may not be as suitable for specific nozzles, since they may also reduce the surface tension of the spray mixture or undermine air induction or Venturi nozzle designs.
Drift suppressing agents may provide other adjuvant functions — such as improved retention and spreading of droplets. Depending on their composition, these adjuvants may also help to improve foliar uptake.
Some Adjuvant Products Contain More Than One Adjuvant Type
You can select an adjuvant product that serves just one purpose or a premix product that performs many functions. The product you select depends on the specific application.
Although a premix may simplify selection and improve compatibility inside the container, they can be a waste of money if they are not the correct solution for the spray mixture. Premixed products may cost more because they offer added convenience. But that convenience can be valuable: some premix adjuvants can help avoid specific compatibility problems, thus eliminating the need to add these materials later.
While a premixed adjuvant may be convenient, they aren’t necessarily better than buying the individual components separately and mixing them yourself. Let your control goals dictate what you buy. Do you need an acidifier if your solution is already at the ideal pH? Do you need a water conditioner if the spray contains very little calcium or magnesium?
Another important factor to consider is whether the ingredients in the adjuvant are at a concentration that will get the job done for your particular application. The ingredients in multifunctional adjuvants are in a fixed ratio relative to the other ingredients. If the ratio is not right, the premix product may not be the best choice for your goals. Know what spray solution problems you want to correct and match them with the right adjuvant product or products.
The Pesticide Label: Suggestions and Silence
Pesticide manufacturers design their formulations to include multiple ingredients (co-formulants). These ingredients are necessary for the manufacturing process, to deliver a stable product, and to ensure robust performance. The specific ingredients differ with product form.
Water-based products typically contain a sufficient amount of a glycol antifreeze agent to stabilize the product for freezing and thawing. The antifreeze agent does not necessarily prevent freezing, but it keeps the formulation stable upon thawing after a hard freeze.
Some pesticide formulations may contain surfactants to modify the wetting and spreading properties needed for foliar application, other formulations may not. If the pesticide doesn’t contain surfactants, the product label may recommend specific tank mix adjuvants.
Other additives in pesticide formulations may include thickeners and chemical stabilizers. Water dispersible granules (WG) include binders and dispersing agents to clump very fine pesticide particles to reduce dustiness and allow the resulting solid granules to redisperse into a fine particle suspension suitable for dilution in the spray application equipment.
However, including adjuvants in some pesticide formulations is impractical. For example, when a pesticide is applied at very low rates, the amount of surfactant needed to modify spray mixtures is often more than the amount of pesticide required. Sometimes the desired adjuvant may be physically incompatible with the pesticide in a highly concentrated form, which could cause formulations to become too thick to measure or pour.
In other cases, an adjuvant preferred for biological activity may not be chemically compatible with a pesticide’s active ingredient that limits shelf stability. For example, organosilicone surfactants are generally not chemically stable in soluble liquid (SL) or suspension concentrate (SC) formulations for extended storage (two or three years). However, they remain quite stable when mixed in the spray tank with many pesticides prior to application. Therefore, pesticide labels often specifically address adding adjuvants to the tank.
A significant benefit of adding your own adjuvants is that it improves flexibility in terms of spray dilutions. Many pesticide products are used with multiple crops, varied climatic conditions, different application equipment types, and changing water use rates per acre. You can customize tank mixtures to assure you add enough adjuvant to modify the spray mixture’s properties to meet the conditions present at the time of the application.
In general, manufacturers can provide greater value in their products when they put some adjuvants into their formulations, but there is limited space in the package for inert ingredients. Active ingredients have a greater value than inert ingredients and adjuvants, so the primary goal is to deliver the most stable and effective concentration of active ingredient in the finished pesticide product. Getting the highest stable concentration of active ingredients in the product allows applicators to handle fewer containers or packages, but not necessarily in ways meaningful for the application.
Another very real reason why manufacturers don’t add adjuvants to their products is that they want to keep their products financially competitive. In this way, pesticide products are like electronic components that are sold in packages with “Batteries not included.” Adding a lot of adjuvants to a pesticide can drive up the cost and may push it out of the market.

Even when manufacturers can add all the adjuvants in their formulated products, there is no perfect formulation that is appropriate for the entire United States and for every growing season. Applicators have to make the ultimate decision to include adjuvants. Only the applicators have access to the information, expertise, and experience about what adjuvants they need based on local conditions.
The critical information applicators consider when making adjuvant decisions could include:
• Water quality parameters such as pH and hardness, which vary drastically around the country.
• The final volume of the spray mix. Adjuvant rates are often determined using a volume-to-volume (%v/v) calculation. Pesticide manufacturers never know where their users will be or where they farm or work. Whether applicators will be using 3 gallons or 100 gallons of spray carrier per acre depends on the industry (agriculture, lawncare, or right-of-way), farming sector (corn or tomatoes), and application equipment (helicopter, ground rig, air blast sprayer, backpack).
• The area to be sprayed. Applicators should consider adjuvant rates on an area basis (adjuvant product per acre) when the carrier is 10 gallons per acre or less to ensure enough adjuvant is present for the pesticide being applied.
• Regional, state, and local differences in targeted pests. The rates and types of adjuvant required may be different between tough-to-control, herbicide-resistant weeds and more susceptible ones. Applicators also should consider application timing and whether crops have less tolerance for a given product.
• Weather. The current conditions can influence how much adjuvant to add. In particular, droughts can greatly influence the adjuvant category and rate to use. Adding an adjuvant to help reduce the droplet’s evaporation rate, or to allow the product to penetrate thicker plant cuticles may be necessary under dry conditions. However, these same properties would be a waste of money in areas that have ample rainfall and high relative humidity.
By the time a pesticide reaches end-users, a manufacturer has invested millions of dollars in research to understand how the product works best. A pesticide label is a national guide for using the product.
Manufacturer labels fall into three categories. The label will:
1. Specifically recommend using an adjuvant
2. Prohibit using an adjuvant
3. Provide no information about an adjuvant
When Labels Recommend Specific Adjuvants
Manufacturers do not give specific brand names for the adjuvants they recommend. Instead, they recommend a specific adjuvant category (such as NIS, COC, MSO, UAN, AMS). For example, the label of a turf herbicide recommends using a nonionic surfactant and further states, “Use only nonionic surfactant on ornamentals.” In any case, if a label recommends a particular adjuvant, then you should use it.
Frequently, recommendations are too generic to assure the desired level of control under specific application conditions, or at a particular location, or for a specific tank mixture. Examples of such vague statements include:
• “To enhance foliage wetting and coverage, an approved nonionic agricultural surfactant may be added to the spray mixture as specified by the surfactant label.”
• “Use only water suitable for spraying. Use an agriculturally labeled nonionic surfactant for all foliar applications. When using surfactants, follow the use directions and precautions listed on the surfactant manufacturer’s label. Use the higher concentration of surfactant in the spray mixture when applying lower spray volumes per acre. The order of addition to the spray tank is water, spray thickening agent (if used), additional herbicide (if used), and [pesticide name]. Surfactant should be added to the spray tank last or as recommended on the product label.”
• “Add surfactant or another nonionic wetting agent to the mixture at label rate.”
• “The addition of 1 to 2 percent dry ammonium sulfate by weight, or 8.5 to 17 pounds per 100 gallons of water (weight/volume), may increase the performance of this product on annual and perennial weeds, particularly under hard water conditions, drought conditions or when tank-mixed with certain residual herbicides. The equivalent rate of a liquid formulation of ammonium sulfate may also be used. Ensure that dry ammonium sulfate is completely dissolved in the spray tank before adding herbicides. Thoroughly rinse the spray system with clean water promptly after use to reduce corrosion.”
A pesticide label may allow a variety of tank mix adjuvants, leaving the final decision about specific adjuvant products to the applicator. In these cases, pesticide dealers or your university extension service may offer helpful recommendations based on past results in your area. This allows dealers and distributors to offer their customers more crop-specific adjuvant solutions.
Always read and follow the pesticide label’s directions carefully. If you have questions, consult the local retailer for added guidance.
When Labels Prohibit Specific Adjuvants
Some pesticide products specifically state using an adjuvant is unnecessary, that an adjuvant will make the product less effective, or that using an adjuvant may cause damage. New pesticide formulations that offer built-in spray drift control technologies may have labels that state that using other adjuvants can increase spray drift.
Product labels may specifically warn users to address specific concerns. For example, adjuvant labels may contain the following warning statements:
• “High relative humidity may increase the risk of temporary discoloration. Use of surfactants is not recommended.”
Buying Adjuvants With Confidence
• “Use only nonionic surfactant on ornamentals. Do not use a crop oil concentrate with [name of product] on ornamentals”
• “Do not add surfactants, additives containing surfactants, buffering agents, or pH adjusting agents to the spray solution when [name of product] is the only pesticide used.”
• “Certain spray tank additives (adjuvants, wetting agents, surfactants), liquid fertilizers, and tank mixtures containing emulsifiable concentrates may reduce the selectivity on the turfgrass. Use adjuvants and spray additives or tank-mix combinations only when your experience indicates that the tank mixture will not result in objectionable turf injury.”
In some cases, getting the benefits you would normally gain from adding an adjuvant might be unnecessary, because the formulated product contains the needed adjuvants. In other cases, using an adjuvant may be antagonistic and lead to less than expected performance or even crop injury.
For instance, one label offers this advice: “Do not add surfactants, additives containing surfactants, buffering agents or pH adjusting agents to the spray solution when [pesticide name] is the only pesticide being applied unless otherwise directed [by this label].” An adjuvant may cause ancillary damage, like burning the foliage of the crop being treated with an herbicide. With newer generations of traits on the horizon, this may become more critical.
Adding surfactants to fast-acting herbicides will only increase the risk of damaging crops. For example, a turf herbicide label states: “Temporary discoloration of some turf types may result from use of surfactants or adjuvants with [herbicide name].
High temperatures and high relative humidity may increase the risk of temporary discoloration. Use of surfactants is not recommended.”
When Labels Are Silent About Adjuvants
A pesticide label may not list an adjuvant or specifically prohibit its use. In these cases, the label is said to be silent on the matter. In the United States, it is not illegal under federal and state pesticide regulations to add an adjuvant when a product label does not prohibit its use.
Pesticide products are often designed for a wide range of applications. Treating some crops may not require adjuvants, while other crops do. Alternately, using an adjuvant for some pesticides may be based on the control of specific pest species. Due to the wide diversity in adjuvant products in the market, it is not always possible to evaluate all of these as a new pesticide product is being developed.
Amending pesticide product labels is time-consuming, since manufacturers must submit label changes to the U.S. Environmental Protection Agency for approval. Often, labels mention adjuvants generically to allow the marketplace some flexibility for possible innovation.
So while it may be legal to use an adjuvant with a pesticide with a label that is silent, you are taking full responsibility for using it. Even the adjuvant manufacturer will specify on their labels that the surfactant “is recommended for use with those pesticides whose label recommends a nonionic wetter/spreader-type adjuvant,” or “Always refer to the label on the product before using [adjuvant product name] or any other product.”
Working outside the scope of the pesticide and adjuvant labels means that you assume responsibility for any unintended consequences of the application. Although it carries risk, your application can produce highly desirable results if you plan it properly.
Ultimately, using adjuvants not mentioned on a pesticide label is a personal decision. You have the opportunity in each application to augment the spray mixture with an adjuvant, because you know:
• How the sprayer is set up
• The characteristics of the water source
• The prominent weeds, insects, and diseases being targeted
• The rates of pesticides and water volume used
• The environmental conditions
• Your previous experiences withthe pesticide and adjuvant
Sometimes a pesticide label may give information that implies a good fit for an adjuvant. For example, a common label states: “When making applications in low relative humidity, set the equipment to produce larger droplets to compensate for evaporation. Droplet evaporation is the most severe when conditions are both hot and dry.”
Another way of accomplishing the label’s instruction to produce larger droplets might be to add a humectant to protect small droplets from evaporation, or a deposition agent that would increase the overall droplet size. These are excellent examples of when planning plays a critical role.
The right professionals can advise whether it is safe and worth the money to add an adjuvant. These professionals include pesticide manufacturer representatives, consultants, local agronomists, and university extension scientists who may have data to support their recommendations or valuable experience with the use of adjuvants in your area.
Growers place a great deal of trust in their network of consultants for good reason: experience. Consultants generally have had more experience (year-in and year-out and over larger regional areas) and have seen both negative and positive outcomes from the use of adjuvants.
Buying Adjuvants With Confidence
In the 19th century, pesticide adjuvants included substances like molasses, sugar, and resins that were used to “stick” sulfur, copper, and lime fungicides to grapes. Kerosene emulsions and soaps derived from whale oil improved the effectiveness of arsenic insecticides.
In the first two decades of the 1900s, insecticides and fungicides used oils. By the 1930s, agricultural researchers began studying the physical properties of water droplets as they related to surface tension and contact angles. The 1940s and 1950s proved to be busy times for adjuvants after nonionic surfactants were introduced. It would not be until the 1980s that AMS and MSO products were introduced. By the 1990s, multifunctional adjuvants were released.
New adjuvant products for pesticide application are developed and introduced each year. Today, there are more than 700 agricultural adjuvants manufactured by more than 40 companies in the United States alone.
The U.S. Environmental Protection Agency (EPA) does not require adjuvant products to be registered. Generally, adjuvant producers formulate their products to contain ingredients that exempt them from crop tolerance limitations set by the EPA. Not being EPA-regulated doesn’t necessarily mean that there are no rules in place that govern adjuvants. Some states, including California and Washington, have extensive state regulations and registration.
American Society for Testing and Materials (ASTM) Section E35:22 also developed a standard terminology with definitions for use with adjuvant products. This enables an improved means for apples-to-apples comparisons when you purchase products.
There also is a trade association —the Council of Producers & Distributors of Agrotechnology (CPDA) — that offers a certification service that reviews adjuvant composition and classification claims. The CPDA provides its seal of approval to an adjuvant product only after undergoing this thorough review process to assure it meets their guidelines and standards.
There are two kinds of adjuvants that will commonly appear in spray mixtures.
The first kind includes post-market or tank-added adjuvants that are not specifically regulated by the EPA and that the grower or applicator adds at the time of application.
The second kind is when that same adjuvant is added as part of a product that is offered for sale. In such cases, the product adjuvant will be called a formulant or inert ingredient, and the EPA has reviewed the adjuvant as part of pesticide registration.
What this really means is that although the federal government does not have a national system for adjuvant registration like it does for pesticides, many of the same adjuvant materials are used in both the registered pesticide formulation and the tank-added adjuvant products. As a result the EPA has reviewed many of those adjuvants at one time or another.
Like any products you buy, it is always a good idea to do your homework before purchasing an adjuvant. Because so much rides on getting the pesticide applied right the first time, it is crucial that you have confidence that both the pesticide and adjuvant labels are accurate, and that your final tank mix will provide the desired benefits under field use.
Here are some questions to consider about registration, legal use, and efficacy.
Questions About Registration
As with any agrichemical, it is important to know whether adjuvant manufacturers will stand behind their products and if their claims about their products’ effectiveness are supported by data. The same goes for adjuvants that are used in medicine, health and beauty aids, and detergents.
Here are two questions about registration that you should ask before selecting an adjuvant product.
1. Is the Adjuvant Registered in California and Washington?
A few states require adjuvant registration. State guidelines vary, but the trend is for more states to regulate them.
California and Washington require a data review and registration process from manufacturers before they allow the adjuvants to be marketed there. Depending on each state’s regulatory requirements, manufacturers can be required to submit the following information (manufacturers registered in California and Washington already address these issues):
• Documentation that the ingredients are acceptable for use on food crops
• Proof of third-party acute toxicology studies
• Evidence that the product will not harm aquatic organisms
• Evidence that the product is effective
• Proof that labels are clearly written and the directions are easy to follow
• Documentation that the labels adhere to the prescribed label formats
• Any precautionary statements to protect applicators
2. Is the Adjuvant Registered With the CPDA?
The CPDA adjuvant certification is a cooperative industry program designed to establish adjuvant product benchmarks. The program relies on ASTM standard classification guidelines.
Adjuvants for Aquatic Uses
Not all adjuvants are labeled for use on aquatic vegetation.
Adjuvants approved for aquatic use must meet specific criteria. Manufacturers must demonstrate that aquatic use will not adversely affect desirable aquatic species. States like California and Washington require data about an adjuvant’s possible aquatic toxicity to fish and aquatic invertebrates. An independent laboratory tests a range of concentrations of the adjuvant to accurately calculate the dose response curve, the LC50 (median lethal dose) for fish, and the EC50 (medium effective concentration) for invertebrates.
Always make sure any adjuvant you use is labeled for aquatic use.
These certifications provide customers confidence that they are purchasing a reputable and reliable adjuvant product.
The CPDA program ensures that adjuvant products meet 17 documentable benchmarks. These benchmarks include that the manufacturers must:
• Demonstrate that the active ingredients listed on the label are present in the product and in the percentages stated in the ingredient statement.
• Verify that the all ingredients were screened for acute toxicological effects on human health.
• Attest that all the ingredients have a tolerance exemption for use in food crops (under the Code of Federal Regulation 40 CFR Part 180) or be approved on food crops by the EPA. The EPA must assess all these materials and then determine that they are safe for their intended use with pesticides applied to food.
The federal code 40 CFR Part 180 organizes inert ingredients into these sections:
• 180.910 — Inert ingredients used pre- and post-harvest; exemptions from the requirement of a tolerance
• 1890.920 — Inert ingredients used pre-harvest: exemptions from the requirement of a tolerance
• 180.950 — Tolerance exemptions for minimal risk of active and inert ingredients
• 180.960 — Polymers; exemptions from the requirements of a tolerance
Manufacturers that meet the CPDA standards for adjuvants may prominently display the CPDA’s certification logo on their labels and in their advertising.
Some adjuvant manufacturers elect to make a stronger statement on their labels. For example, “[Name of product] is certified by the Council of Producers & Distributors of Agrotechnology (CPDA), which ensures consistency in product performance. CPDA certification assures users that the adjuvant product meets the claims indicated on the label; that ingredients used in the product meet EPA regulations for approved ingredients for use in pesticide tank mixes; and that the product is of the highest level of quality and reliability.”
Using the adjuvant certification is an excellent way of knowing that the manufacturer voluntarily solicited CPDA review of their product to provide their customers confidence in the quality and utility of their product.
A current list of certified products (along with their labels and material safety data sheets) are available on the CPDA website, cpda.com.
Question About Legal Use
Here is a question about legal use that you should ask before selecting an adjuvant product.
1. Is There an Exemption Statement?
Look at the label to see if any of the ingredients are exempt under 40 CFR Part 180. If it is, you should find a statement like this on the label:

“Ingredients are exempt from tolerance under 40 CFR Part 180.” The label may also state: “All ingredients are accepted for use under 40 CFR Part 180.”

If adjuvant products do not include these statements on their labels, ask the manufacturer whether the products can be legally used on food crops. Without the manufacturer’s assurance, you should not use an adjuvant product on food crops.
Questions About Efficacy
Here are three questions about efficacy that you should ask before selecting an adjuvant product.
1. What Do Manufacturer Reps or Dealers Say?
It seems logical to get input from a pesticide manufacturer’s sales or research representative. If these representatives don’t know the answers, they have access to other professionals in their company who can answer your questions about using adjuvants with their specific products.
2. Is the Seller Reputable?
It can be important to buy products from people and companies who have a vested interest in your operation. You do business with them for a number of reasons including price, expertise, trust, and prior history. It is reasonable to expect good service and well-reasoned opinions about adjuvant selection and use if you’ve been happy with past recommendations.
3. Do Data Support Efficacy Claims?
Always consider your desired outcomes when choosing an adjuvant. The least expensive adjuvant may not always deliver the best value. Dealers, distributors, and adjuvant producers can often provide past field data from their customers or university trials to support their claims about the expected benefits of the adjuvants they recommend.
Adjuvant manufacturers should be able to provide data that show their products have been tested in the greenhouse and evaluated under field conditions. Contact your state’s extension service to see if they have collected efficacy data in university research trials.
Without some performance data to back up your purchase decision, you are blindly betting that the cost of the adjuvants you are applying will, in fact, provide a benefit. It’s always better to make an educated choice when data supports using an adjuvant.
Pay particular attention to data that provide:
• Side-by-side comparisons of competing products.
• Efficacy results that compare a pesticide’s efficacy with and without an adjuvant.
• Data from replicated trials. Such data are better than data from demonstration plots, which are better than data drawn from testimonials. Replicated trials give scientific credence to the conclusions drawn.
• Evidence that the product provides consistent results over a number of years and environments.
It’s always best to test an adjuvant by running your own field trial. It doesn’t have to be large or complicated. Spray one load with the adjuvant and one load without (or with an alternative adjuvant), and then evaluate performance within the first few weeks. You can judge yourself whether adding the adjuvant was worth the money.
Reviewing the Adjuvant Label
Adjuvants normally come with one-page labels that resemble pesticide labels. The quality of adjuvant labels has improved, largely because of the registration programs in California and Washington that require labels to have specific information on them. However, products that are not registered in those states may appear much simpler than the one presented below.

The active ingredient section can, at times, be confusing and misleading. However, the example in the COC label indicates that 100 percent of its ingredients are active ingredients. Most of the product is an oil-based active ingredient that provides penetration properties, while the remainder is a surfactant that helps spray droplets spread over the leaf surface. This is an example of a label that is straightforward and easy to interpret.
In the example shown in the illustration below, products A and B are sold as 80-20 NIS adjuvants. Both products state that active ingredients make up 80 percent of the product and inactive ingredients make up 20 percent. The general assumption is that both products are similar based on the ingredients.
But look closer at the differences between these products. Product A has 40 percent surfactant load in its formulation, while product B has 20 percent surfactant.
Product A has twice as much surfactant, which is the ingredient that actually gets the job done in the spray tank. So while each is an 80-20 adjuvant, there are large differences in what you pay for.
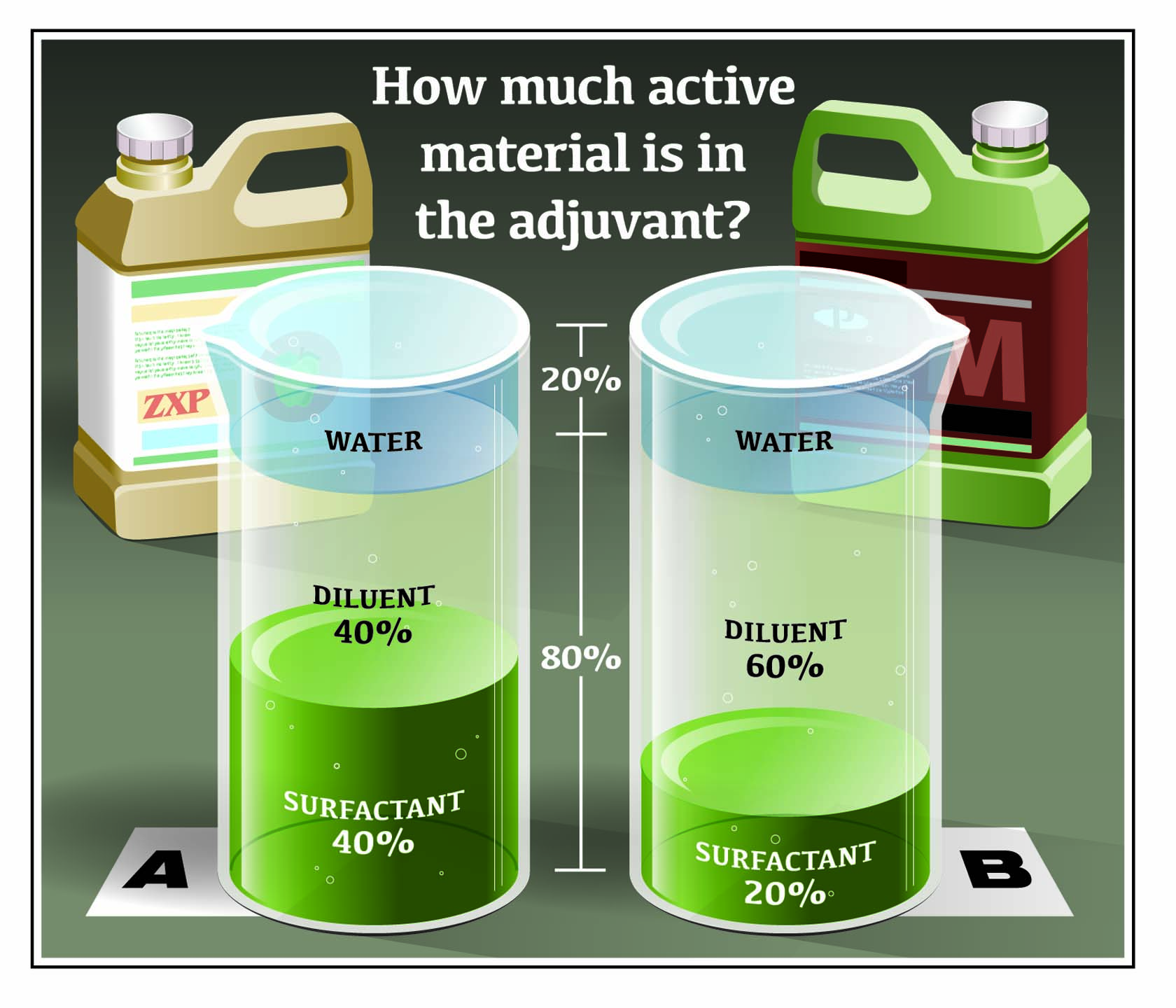
Review the product label for the adjuvants you are considering so that you can determine why there may be a difference in the cost per acre of the products or how well they may perform for your specific application.
The illustration below is another example. An adjuvant label states that the product is a 90-10 blend — the formulation is composed of 90 percent active ingredients and 10 percent carriers. As you can see from the ingredient statement, the manufacturer has added surfactants, humectants, and fatty acids to the list of active ingredients. Notice that the label indicates that 70 percent of the formulation is surfactant-based. Labels such as this one make it easy to see exactly what you are paying for.

Rates
Adjuvant labels calculate recommended tank mix rates using either a volume-to-volume ratio (v/v) or product rate per 100 gallons of water. A COC label indicates the user to mix a 0.5 to 1.0 percent ratio per 100 gallons, which is equivalent to 4 to 8 pints.
There are labels that only provide the percentage ratio without making the conversion in pints for you. Let’s use the COC to figure out how the 4 to 8 pints were calculated. To begin with there are 8 pints per gallon.
100 gallons x 8 pints/gallon = 800 pints
0.5% = 0.005 and 1.0% = 0.01
0.005 x 800 pints = 4 pints and
0.01 x 800 pints = 8 pints
Scientists prefer replacing volume ratios with a fixed rate per acre when the volume of water used as the spray carrier is significantly reduced, especially when using less than 10 gallons of carrier per acre. For example, the label of one COC indicates that aerial applications and low volume sprays should use 1 to 4 pints per acre, which is a higher dose of COC than if we calculated the rate at 0.5 to 1% (v/v) at an application volume of only 10 gallons per acre.
There comes a point when the volume of water is so low that there is simply not enough of the adjuvant present in the tank to maximize the transfer, rate, diffusion, and uptake when you use the volume-to-volume rates. In effect, you are cutting the use rate of the adjuvant as the water volume is reduced.
Some adjuvants define low water volume use rates. An MSO concentrate indicates that when applying more than 5 gallons per acre by ground equipment, use 4 to 8 pints per 100 gallons. If using less than 5 gallons per acre, use 6 to 8 ounces per acre. When a label does not give instructions about what constitutes a low volume of water, check with the adjuvant supplier or manufacturer to see what rates they would use given your specific needs.
The more difficult challenge is to know what part of the rate range to use. Adjuvant labels are seldom helpful.
Check the pesticide label first, but this may not help. If the pesticide label calls for a specific category of adjuvant but does not provide a rate, ask your supplier for a recommendation. If you can’t draw on the knowledge or experience of these individuals, then initially select the middle to the high end of the rate range. After you become familiar with the product, you can adjust the rates.
You can change the rates of anyadjuvant based on prior experiences. You might use the higher rate of a drift control adjuvant on a windy day but cut back on days with less wind. Spraying during drought conditions after plants have hardened may require you to use more of an oil adjuvant to deal with the thicker layer of wax on the leaf surface.
One of the last issues to address is the mixing order. The first rule is to follow the instructions on pesticide labels, which normally state to add adjuvants last. For example, the instructions might read: “Add pesticides as directed by labeling or in the following sequence: dry flowables or water dispersible granules, wettable powders, flowables, solutions, emulsifiable concentrates, and add [adjuvant name] last and continue agitation.”
But a pesticide or adjuvant label may recommend adding the adjuvant in a different order, so read all label directions before mixing products. Some adjuvants (such as pre-emulsified, predispersed or emulsified) may not be as sensitive and can be added at different points in mixing depending on convenience.

Conclusions
Making the winning play in a pesticide application requires pregame preparation (proper identification, growth patterns, and vulnerable life stages). The better you prepare, the more likely you are to win.
If in a football game your lineman doesn’t put a solid block on the opponent’s linebacker, your back might run for 5 yards. But if the lineman makes the block, that 5-yard gain could be a touchdown. A 5-yard run may seem adequate, but it is not what it might have been if the play had been properly executed.
Like that 5-yard run, the results of a pesticide application may be acceptable. But adding the right adjuvant might have been like the lineman making that block. Running interference for the spray droplet, adjuvants can help the spray mixture score every time.
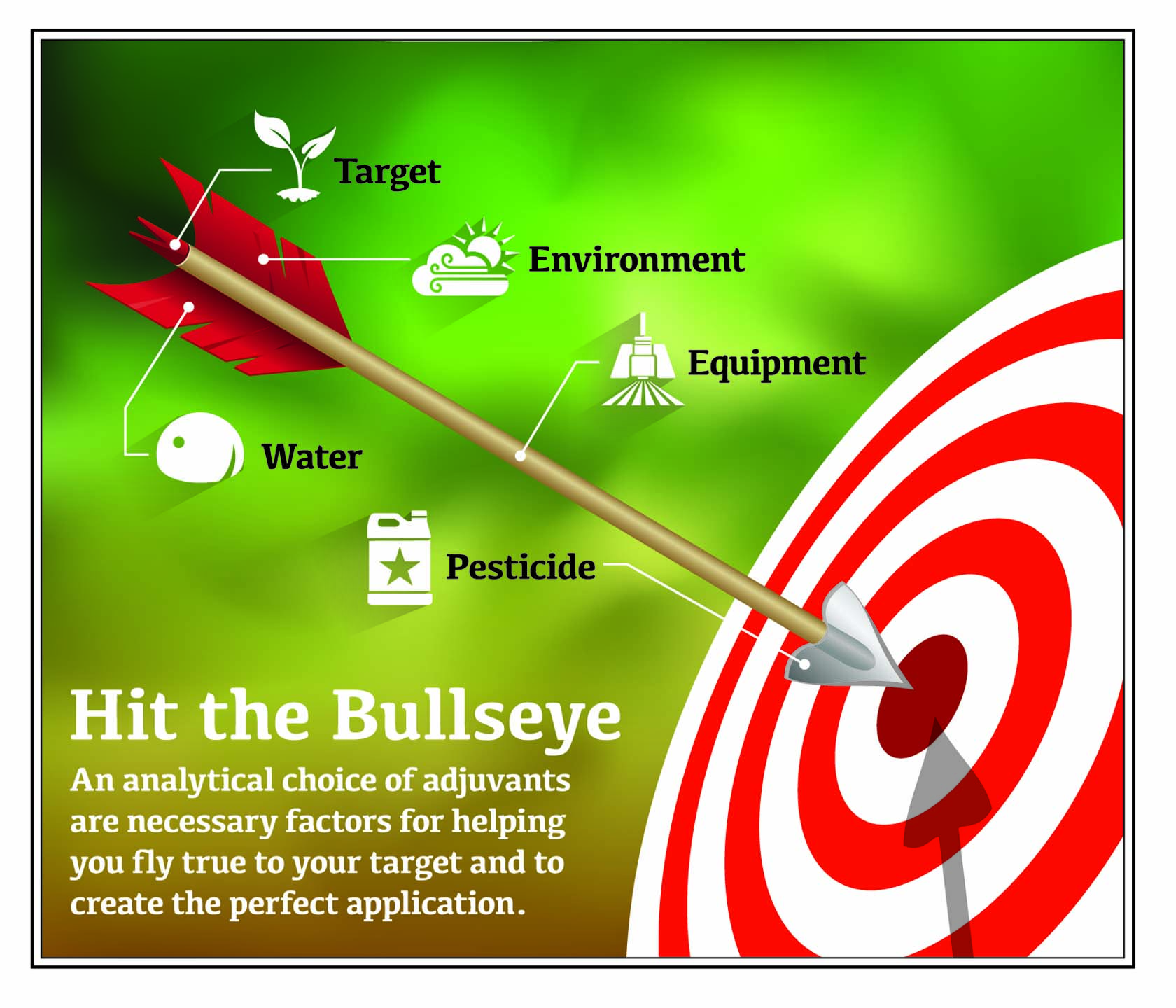
Acknowledgements
Tom Bauman, Purdue University
Janna Beckerman, Purdue University
Larry Bledsoe, Purdue University
Bruce Bordelon, Purdue University
Scott Bretthauer, University of Illinois
Steve Brewer, President, Brewer International
Jim Brosnan, University of Tennessee
Ernie Brumbaugh, Amway Corporation
Dan Childs, Monsanto
Jim Colias, Matt’s Lawncare and Landscaping
Gary Crum, Lawn Tamer
Greg Dahl, Winfield Solutions
Jim Donahoe, Aquatic Weed Control
Steve Dlugosz, Heartland Cooperative
Dan Egel, Purdue University
Moe Finke, Consultant
Mike Foley, Superior Forestry Service, Inc.
Brad Fritz, United States Department of Agriculture Agricultural Research Service
Dave Gardner, The Ohio State University
Matt Giese, Syngenta Professional Products
Dave Hillger, Dow AgroSciences, LLC
Mark Kinsey, Crop Production Services
Travis Legleiter, Purdue University
Linda Marshall, Biosorb
Patrick McMullan, United Suppliers, Inc.
Dallas Peterson, Kansas State University
Jim Reiss, Precision Laboratories
Steve Roehl, West Central Cooperative
Gene Schellhorn, Pentair
Brent Sellers, University of Florida
Solito Sumulong, Winfield Solutions
George Watters, Winfield Solutions
Scott Tann, Huntsman
Kent Woodall, Rosen’s Inc.
Disclaimer
This publication is intended for educational purposes only. The authors’ views have not been approved by any government agency, business, or individual. The publication is distributed with the understanding that the authors are not rendering legal or other professional advice to the reader, and that the information contained herein should not be regarded or relied upon as a substitute for professional consultation. The use of information contained herein constitutes an agreement to hold the authors, companies or reviewers harmless for liability, damage, or expense incurred as a result of reference to or reliance upon the information provided. Mention of a proprietary product or service does not constitute an endorsement by the authors or their employers. Descriptions of specific situations are included only as hypothetical case studiesto assist readers of this publication, and are not intended to represent any actual person, business entity or situation. Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind by Purdue University. Individuals using such products assume responsibility for their use in accordance with current directions of the manufacturer. Any picture of a product used in a way not intended by the manufacturer is neither endorsed nor condoned by the authors nor the manufacturer.
Purdue Pesticide Programs offer a number of publications on related topics to help you manage your operations better. All publications are available from The Purdue Extension Education Store:
edustore.purdue.edu
(888) EXT-INFO (496-4636)
The Impact of Water Quality on Pesticide Performance (PPP-86). Understand how your mixing water influences pesticides.
Pesticides and Formulation Technology (PPP-31). Learn the details of pesticide formulations and how to use them effectively.
Measuring Pesticides: Overlooked Steps to Getting the Correct Rate (PPP-96). Understand effective measuring techniques that ensure success.
Poly Tanks for Farms and Businesses (PPP-77). Learn more about selecting and using poly tanks.
Fiberglass Tanks for Storage, Transport, and Application: Designing Your Customized Tank (PPP-93). Get all the necessary information about fiberglass tanks before you buy.
Steel Tanks for Storing and Transporting Pesticides and Fertilizers (PPP-105). Get all the necessary information about steel tanks before you buy.
Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind by Purdue Extension. Individuals using such products assume responsibility for their use in accordance with current directions of the manufacturer.
Find out more at
The Education Store edustore.purdue.edu
September 2014