Anhydrous Ammonia

Fred Whitford, Director, Purdue Pesticide Programs
John Rebholz, Director of Safety & Education, Illinois Fertilizer and Chemical Association
Jim Camberato, Extension Agronomist, Purdue University
John Obermeyer, Integrated Pest Management Specialist, Purdue University
Mike Titus, Safety and Risk Coordinator, Co-Alliance
Jamie Southard, Vice-President, EHS and Facilities, The Equity
Danny Starke, Agricultural Ammonia Specialist, Office of Indiana State Chemist
Shawn Lambert, Safety & Risk Manager, Co-Alliance
Kevin Leigh Smith, Continuing Lecturer and Communication Specialist, Purdue Agricultural Sciences Education and Communication
Preventing a Release Is Far Better than Reacting to One………………. 4
Chemical Characteristics of Ammonia Worth Knowing ………………… 6
Liquid Under Pressure ………………………………………………………………. 6
Easy to Detect With Its Distinctive Odor ………………………………………… 6
Strong Affinity for Water …………………………………………………………….. 6
Lighter Than Air ……………………………………………………………………….. 8
A Caustic Material …………………………………………………………………….. 8
Freezing and Boiling Points ………………………………………………………… 9
Protecting Your Facility with Inspections and Signs…………………….. 10
Emergency Contact Information Posted at Site Entrance …………………. 10
Delivering Anhydrous Ammonia to the Facility ……………………………. 12
Bulk Storage Tanks ………………………………………………………………….. 16
Transferring Anhydrous Ammonia to Application Tanks …………….. 20
Understanding Nurse Tank Valves and Decals ………………………………. 22
Filling Nurse Tanks From the Riser ………………………………………………. 27
Moving Nurse Tanks To and From the Riser …………………………………… 31
Transporting Anhydrous Ammonia In and Out of the Facility ……… 34
Applying Anhydrous Ammonia ………………………………………………….. 40
Human Error Is Behind Most Anhydrous Ammonia Releases ……… 46
Preparing Before an Emergency Occurs ……………………………………. 52
Employee Training …………………………………………………………………… 52
Fire Department Training ………………………………………………………….. 53
Reacting During a Medical Crisis ………………………………………………. 54
Steps for Dealing With Injuries ………………………………………………….. 54
Four Scenarios ……………………………………………………………………….. 58
Play It Safe ……………………………………………………………………………. 58
Responding to the Vapor Cloud …………………………………………………. 60
Mandatory Reporting of Anhydrous Ammonia Releases …………….. 66
Conclusions ……………………………………………………………………………… 68
Acknowledgments …………………………………………………………………….. 70
Disclaimer …………………………………………………………………………………. 71
Anhydrous ammonia is available from hundreds of agricultural retailers for their farm clientele who use this fertilizer on their corn acreage. While driving through Indiana’s farmland in late fall or spring, it is a common sight to see retailers and farmers pulling anhydrous ammonia nurse tanks to and from fields. During peak times, retailer lots that were once filled with nurse tanks and applicators (tool bars) stand empty as every working piece of anhydrous ammonia equipment is pressed into service.
Using anhydrous ammonia offers many benefits:
• The cost per pound of actual nitrogen with anhydrous ammonia is less than urea, UAN 28-32%, and aqua ammonia.
• Anhydrous ammonia is 82 percent nitrogen, so transportation costs per unit of nitrogen are much lower than other forms of nitrogen.
• It is a popular form of nitrogen recommended for fall application in Indiana and other Midwest corn-producing states
However, there are major risks associated with using anhydrous ammonia. We probably have all seen, read, or heard about anhydrous ammonia releases. A “white vapor fog” sweeps across the landscape and emergency responders are called out to deal with the escaped gas.
Depending on the direction of the release, roads in and out of the area may be blocked and the people near the release site evacuated. In other situations, local authorities may order people to shut windows and shelter in place until they give the all-clear notice.
Many things can cause releases: a leak or discharge from a ruptured hose at the filling riser or during field applications, a release from an overturned anhydrous ammonia tank in a road accident, or discharges from improperly connected hoses.
Firefighters responding to the incident have only a short time to spray water onto the anhydrous ammonia cloud to mitigate the vapor and reduce its movement. While most anhydrous ammonia releases are safely dealt with, there are documented cases in which local residents and firefighters have become permanently disabled or killed when engulfed in the vapor cloud.
When a release is major, local hospitals can quickly become overwhelmed by injured people seeking emergency care. What’s more, ammonia releases that injure or kill may lead to lawsuits that can drag through the courts for years.
It’s an undeniable fact that anhydrous ammonia has what we might call “Jekyll and Hyde” attributes. Anhydrous ammonia is a low-cost and readily available nitrogen fertilizer. However, because of its potential hazards to humans and the environment, those who store, transport, and use anhydrous ammonia must be constantly reminded to focus their attention on even the smallest of safety details.
Ammonia training — and retraining — needs to be provided to part-time seasonal workers, newly hired recruits, tenured veterans who work outdoors, and even those who work behind customer counters at retail facilities or on farms. A constant focus on anhydrous ammonia safety helps ensure that everyone fully appreciates their role in protecting themselves, their coworkers, and the community. Farmers and farm employees who handle ammonia also would benefit from this training. In some states, farmers and farm operators are now required to have ammonia safety training.
This publication provides you with a better understanding of the hazards of anhydrous ammonia, how to safely work around this product, and what you should do in the event of a release. Throughout the publication, we have Do You Now Understand segments. We hope that correctly answering the questions in these segments will help you to better understand the concerns of working around anhydrous ammonia.
Chemical Characteristics of Ammonia Worth Knowing
If you want to know why there are so many regulations surrounding anhydrous ammonia, then it helps to understand its chemical and physical properties. You don’t have to be a chemist or need to be familiar with complex chemical reactions to understand the chemical properties and reactions associated with anhydrous ammonia.
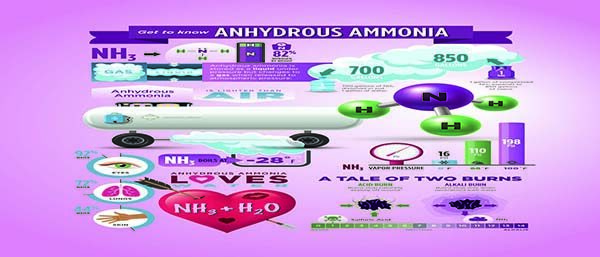
Let’s start with anhydrous ammonia’s structure. You may see or hear anhydrous ammonia referred to by its chemical formula: NH3. The formula tells us that anhydrous ammonia molecules contain three parts of hydrogen (H) for every one part of nitrogen (N). But the weight of the N atom makes up 82 percent of the anhydrous ammonia compound. This makes anhydrous ammonia a concentrated nitrogen product, 82-0-0.
LIQUID UNDER PRESSURE
At ambient temperatures and pressures, anhydrous ammonia is a gas. It is transformed into a liquid when it is compressed under pressure in an approved storage tank. When released into the air, anhydrous ammonia converts back to a gas (the term vapor is often used interchangeably with gas). An interesting fact is that one cubic foot of liquid anhydrous ammonia, contained under pressure, produces 850 cubic feet of vapor when released, an expansion rate of 850 to 1.

EASY TO DETECT WITH ITS DISTINCTIVE ODOR
When released into the air, anhydrous ammonia is colorless, but the odor is quite recognizable: it smells like ammonia, a common household cleaner. The odor is often described as “sharp” or “pungent.” At high concentrations, it can be described as “suffocating.”
Most people can detect ammonia’s distinctive odor at between 5 and 50 parts per million according to OSHA. That is well below the levels (greater than 220 parts per million) that can cause irreversible harm to humans.
STRONG AFFINITY FOR WATER
The word anhydrous means a substance without water. For this reason, anhydrous ammonia has a strong affinity for water — whether in the atmosphere, in the soil, or in contact with your body. Anhydrous ammonia takes water from anything it comes in contact with. This is why anhydrous ammonia can seriously damage your skin, which is 44 percent water. Even more serious is the potential damage to eyes (92 percent water) and lungs (72 percent water).
This is why it is important that there are always emergency water tanks available. There are 5-gallon water tanks on nurse tanks and larger emergency soak tanks that an exposed person can immerse in at the retail facility.

LIGHTER THAN AIR
Anhydrous ammonia vapor is lighter than air, which means it moves up into the atmosphere from the point of its release. However, high relative humidity (like we commonly experience in the Midwest) traps the vapor. When ammonia encounters moisture in the air, the vapor becomes heavier and hangs lower to the ground.
Emergency responders often describe what they call the upside-down J phenomenon of an anhydrous ammonia gas cloud. When released from the top of a tank, the vapor goes straight up into the atmosphere. When the vapor rises 50 to 75 feet, the anhydrous ammonia cloud starts to curl over and flatten out as it picks up atmospheric moisture (getting heavier in the process). This phenomenon has misled people into thinking it was safe to reenter release areas too soon and suffer severe injuries.
A CAUSTIC MATERIAL
Anhydrous ammonia has a pH of 11, which is just slightly less alkaline than bleach, which has a pH of 12. Remember the chemical formula for anhydrous ammonia: NH3. When it absorbs water (H2O), anhydrous ammonia removes a hydrogen atom from water to take on the more stable form of NH4+ (ammonium).
When this process takes place in the soil, this reaction is good, because the nitrogen remains in place for the plant’s use. But when that reaction happens in the air because of a release near you, removing the hydrogen atom from water leaves behind a molecule of hydroxide (OH-), which is very caustic to skin, lungs, and eyes. Water is the only immediate first aid for ammonia exposure.
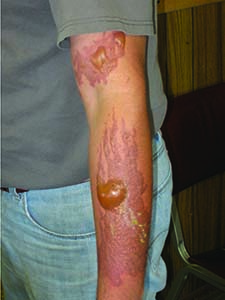
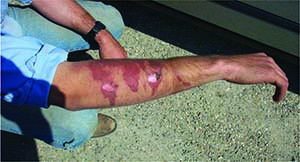
Anhydrous ammonia injury to the skin.

FREEZING AND BOILING POINTS
Anhydrous ammonia boils at -28°F. This is why it turns into a vapor when it is released at ambient temperatures. The freezing point of anhydrous ammonia is -108°F, which means that when anhydrous ammonia changes from liquid to a vapor, it is at first very cold, which can further damage human and plant tissues.

Protecting Your Facility with Inspections and Signs
The description of its chemical properties provides ample evidence for why there is such an investment in specialized infrastructure to store, dispense, and transport anhydrous ammonia. Because of this, major anhydrous ammonia releases at retailer sites are fairly rare, even though the industry moves hundreds of thousands of tons of ammonia each fall and spring. Tanks, plumbing, and additional safeguards constructed according to national standards and government regulations are partially responsible for making releases rare.
Furthermore, initial installations undergo routine inspections by independent industry consultants, company-employed safety and risk coordinators, and government inspectors who help ensure continued compliance with regulations and company safety practices. Ultimately, the safe use of anhydrous ammonia depends on employees who pay close attention to every aspect of the process and report potential problems.
EMERGENCY CONTACT INFORMATION POSTED AT SITE ENTRANCE
One of the first steps in that process is that every facility should place an emergency sign at the most prominent entrance where emergency responders (including fire, police, and medical professionals) would enter. In Indiana, emergency signs must be placed at every entrance.
Each emergency sign provides the name of the location and its 911-approved address. The sign also provides the names and phone numbers (including area codes) of those with the knowledge to deal with an anhydrous ammonia release at that facility.
Emergency signs posted at entrances also provide legends that inform emergency responders what the pipes carry and what the valves control. In Indiana, pipes painted white for reducing heat mean they are carrying anhydrous ammonia either as a liquid or vapor. Valves painted orange indicate the lines are carrying liquid. Valves painted highway yellow indicate vapor lines.


Delivering Anhydrous Ammonia to the Facility
Drivers who deliver anhydrous ammonia to retail facilities are expected to conduct their business in a professional and safe manner — no different than anyone else working around ammonia. Two critically important tasks that drivers must complete occur at the beginning and at the end of the delivery process.
First, drivers should not fill storage vessels more than 85 percent of their capacity. This allows room for the anhydrous ammonia in the tank to expand as ambient temperatures rise. Storage tanks typically hold anywhere from 12,000 to 30,000 gallons of liquid anhydrous ammonia — 45,000- and 60,000-gallon tanks are also common. Each storage tank has a float gauge that helps determine how full it is (by percentage).
A transport truck normally carries 8,000 gallons (or roughly 20 tons) of anhydrous ammonia; anhydrous ammonia weighs 5.14 pounds per gallon. Since a driver can fill a storage tank to 85 percent of its rated capacity, a 12,000-gallon storage tank could hold one delivery truck worth of ammonia.

A MAJOR DELIVERY SCREWUP
Many retail facilities that handle anhydrous ammonia also handle propane. There are serious consequences if someone mistakenly pumps ammonia into a propane tank.
The fittings on propane tanks are made of brass and copper. The fittings on ammonia tanks are made of mild steel and stainless steel. Putting ammonia in a propane tank creates pinholes in the brass and copper fittings.
You want to know with certainty that delivery drivers and employees are putting ammonia and propane in the right tanks. If the truck in front of the propane tank has ammonia written on its side, or vice versa, then employees have the responsibility to tell the drivers to stop and make sure they are filling the correct tank.
It is better to ask and be wrong than to ignore what your eyes are telling you.

After drivers fill the storage tanks and shut down both their trucks and the storage tanks, they still have one critical task: Do not allow any liquid trapped in the hose to escape into the environment. There can be as much as 1 gallon of liquid left in the delivery truck’s hose, which will turn from a liquid to a gas if drivers disconnect the hose at this point.

When drivers unload ammonia, the hoses are connected between the trucks and the tanks, and are under pressure. When drivers complete their product transfers, they must relieve the pressure in the hose by opening the bleed off. Drivers leave the hoses connected under pressure, but opening bleed-off valves will drain what is left in the connection into a nearby enclosed water tank designed specifically for this purpose.
It’s common to use a 275-gallon caged minibulk tank to collect this bleed-off anhydrous ammonia. The anhydrous ammonia is absorbed into the water. This leaves little product in the hose when it is disconnected from the fitting at the bulkhead.
It is important to close the valve to the bleed-off tank when it is not being used — leaving the valve open can cause back pressure and suck water back into the ammonia piping. The bleed-off„water contains nitrogen, which can be applied at agronomic rates to cropland — either by itself or along with other fertilizer or chemical applications.
It is equally important to recognize that the bleed-off water can become saturated with ammonia. You should change the water several times during the season considering that as many as 10 transports come to a facility each day during the busy season.

Delivery drivers should be expected to:
• Know the correct traffic patterns for unloading at a storage site.
• Have a valid CDL and hazardous materials endorsement.
• Set the brakes on their vehicles and chock the wheels.
• Know where the emergency shut-off is and how to operate it.
• Check to ensure clean water is available in the event of an emergency.
• Wear long-sleeved shirts and pants, gloves that are rated for anhydrous ammonia, and nonvented or indirect vented goggles (at a minimum).
• Make sure the bleed off-system is shut off before starting the transfer pump. Bleed-offs need to be vented. There have been fatalities when the bleed-off containers were not vented and became pressurized.

• Connect both the vapor and liquid hoses to bulkheads before starting the transfer process.
• Open valve-side systems first to allow the vapor to equalize between cargo tank and storage tank.
• Fill storage tanks to no more than 85 percent of their capacity.
• Bleed-off any remaining ammonia in the hoses.
• Stay with their trucks until the process is totally complete.
• Monitor and observe the transfer activities from the ground and not from inside their cabs.
BULK STORAGE TANKS
Tanks used to store anhydrous ammonia are basically heavy, thick submarine-like tanks. Many of these tanks were built in the 1960s and 1970s. A storage tank contains the largest volume of anhydrous ammonia at the facility, which is why maintenance on the tanks is so vital. That maintenance includes keeping them painted and keeping the pipes free of corrosion.
Storage tanks must be built to withstand internal pressures up to 250 pounds per square inch (psi). However, anhydrous tank manufacturers actually construct them with a 4-to-1 safety factor, meaning they can withstand pressures higher than 250 psi.
These tanks have a series of external and internal valves. There are pressure relief valves that open in the event the pressure gets too high within the tank. There are excess flow valves that can shut down the flow of liquid anhydrous ammonia in the event of a plumbing failure or hose rupture. There are back check valves that allow product to move in only one direction. All of these built-in safety devices are yet another step to prevent major releases.
It is important to familiarize yourself with the locations of each of these safety devices and how they operate. Each has its own specific function. Knowing what those functions are and how they operate within the system allows a quicker response in the event of an emergency.
Can you find these at your facility?
• Crash protection around the storage tank to protect tanks, piping, and valves against the impact from moving vehicles and equipment.
• Pull-away protection in case hoses are not disconnected from the bulkhead or refilling station after refilling.
• Color-coded valves and piping legend that indicates whether it is liquid or vapor in the lines.
• “Anhydrous Ammonia” or “Caution-Ammonia” painted on the side of the storage tank. The National Fire Protection Association (NFPA) diamond also is required to be painted or decaled on the tanks. The NFPA diamond’s multiple colors gives first responders quick information about the hazards posed by the anhydrous ammonia.
• Manual valves to turn off tanks.
• Liquid level fill gauge.
• Bleed-off tank.
• Emergency shut-off system on the transfer truck and storage tank.
• Two tanks that hold at least 150 gallons of water that a person can physically jump in if that person has been exposed to ammonia. The tanks should be able to hold the largest employee working at the facility.

Transferring Anhydrous Ammonia to Application Tanks
The location where you fill application nurse tanks with anhydrous ammonia is an elevated area commonly referred to as the riser, rack, or filling station. Many locations have a liquid pump that transfers anhydrous ammonia from the larger storage tank via pipes coming up through the center of the riser. At the riser, the liquid anhydrous ammonia enters hoses that lead to primary shut-off valves, commonly called hose-end valves.
This elevated platform allows one person working on the riser to access the valves on top of the nurse tanks to connect the hoses. Most risers can fill four nurse tanks at one time — two on each side. Filling time varies, but at newer facilities, it can be as little as 10 minutes per nurse tank.
Nurse tanks are built to exact specifications that are dictated by federal regulations and industry standards (American Society of Mechanical Engineers, or ASME). This is a good thing when you consider that these tanks are pressurized vessels on wheels carrying hazardous materials. Older nurse tanks have capacities of about 1,000 to 1,450 gallons. Today with larger farm equipment, it is common for tanks to hold up to 3,000 gallons of anhydrous ammonia.
Each nurse tank must have a clearly legible data plate. If a nurse tank does not have a clearly legible data plate, the tank must undergo a series of tests to ensure its safety and integrity.
Farmers are exempt from U.S. Department of Transportation (USDOT) regulations dealing with commercial drivers licenses (CDL) and hazardous materials endorsements when they transport 3,000 gallons or less of anhydrous ammonia in any combination. Be aware that these rules may be more stringent when crossing state lines.


UNDERSTANDING NURSE TANK VALVES AND DECALS
In order to safely handle anhydrous ammonia, here are some helpful facts about nurse tanks.
Most nurse tanks are painted white. This reflects sunlight to reduce the tank’s internal heat, thus, minimizing the pressure within the nurse tank.
Each nurse tank is required to have four different types of decals. — (1) a 1005 placard, (2) an “ANHYDROUS AMMONIA” decal placed on all four sides (even on side-by-side nurse tanks), and (3) an inhalation warning decal placed on each side. In Indiana, (4) a safety information decal placed near the emergency water tank is also required. Emergency responders use the placards and decals during incidents involving anhydrous ammonia.
Nurse tanks have four legs that are secured to its running gear. The running gear needs to be adequately sized for the tanks and weight placed on them. Because they are implements of agriculture or implements of husbandry, nurse tanks are not required to have brakes.
However, many newer nurse tanks are equipped with brakes. Slow-moving vehicle (SMV) emblems or metal signs are affixed to nurse tanks. SMV signs inform those approaching that the equipment in front of them is moving at slower than normal traffic.


The valves on top of nurse tanks are enclosed in a roll cage to protect them in case the tank is involved in a roll-over accident.
While the order of the valves can vary, nurse tanks are constructed with similar valve configurations and are consistently labeled.


Here are the six kinds of nurse tank valves and their functions.

1. The Liquid Withdrawal Valve
The liquid withdrawal valve, commonly known as the farmer’s valve, has a tube that goes to the bottom of the tank that allows the withdrawal of liquid anhydrous ammonia. This valve is always painted orange, which indicates liquid.
The withdrawal valve incorporates an excess flow assembly that shuts the system down if the flow rate exceeds a certain number of gallons per minute (GPM). That’s generally 45 to 60 GPM, depending on the size of the container’s opening. In the event of a release exceeding those amounts, the spring in the excess flow assembly snaps shut. The excess flow assembly may not work if it is rusted or hampered by other debris, or has degraded O-rings. In addition, the excess flow assembly may not activate to stop a release if a hole develops in a hose where full flow is not achieved.
REPAIRS BEYOND YOUR WELDING SKILLS
Keep in mind that nurse tanks are actually pressurized vessels that carry hazardous material.
The fact that the material is stored under pressure requires that any repairs to the tanks must be performed by professional welders who have special certifications and experiences. Never weld anything to the actual container unless you are a welder with an R stamp certification.
2. Vapor Fill Valves
Vapor fill valves are used to add liquid anhydrous ammonia and to equalize vapor. They are painted yellow to indicate that the valve extends only into the vapor space.
When filling the tanks, either valve will work for pumping liquid anhydrous into the tank. If nurse tanks are involved in an accident that results in the tanks being upside down, then the valves will operate in a different space — the orange valve will be in the vapor space and the yellow valves will be in the liquid space. When that happens, the tank must be turned upright before you can remove liquid anhydrous.
3. Fixed Liquid Level Gauges
The fixed liquid level gauge is commonly called the spit gauge. It is a bleeder valve that has a dip tube that extends inside the tank to the 85 percent liquid fill line. When the liquid hits the 85 percent level, the liquid ammonia pushes up through the tube and creates a “spitting” action that can be observed. In Indiana, the fixed liquid level gauge is the official way to fill nurse tanks to 85 percent.
4. Float Gauges
Float gauges are visual indicators of the liquid levels in nurse tanks. However, you should only use float gauges as a reference, because internal floats can easily be damaged.
5. Pressure Gauges
Pressure gauges typically read from 0 to 400 pounds per square inch (psi). You should not rely on pressure gauges to determine the fill status of the tank. Remember: these gauges only tell you pressure, not fullness.

6. Pressure Relief Valves
Pressure relief valves (commonly referred to as a PRV or pop-off‚ valve) opens the tank if pressure inside the tank exceeds 250 psi.
FILLING NURSE TANKS FROM THE RISER
Employees on the riser have one main job: to safely fill the nurse tanks. There are three golden rules that people charged with filling nurse tanks need to keep at the top of
their minds at the start of their shifts before they open the first valve.
Golden Rule Number 1
Every person on the riser should wear the appropriate safety equipment at all times.
In addition to wearing long pants and long-sleeved shirts, personal protective equipment approved for anhydrous ammonia includes gloves and chemical splash goggles.
SAFETY EQUIPMENT FOR ANHYDROUS AMMONIA USE
When working with anhydrous ammonia, it isn’t enough to just have gloves and goggles — be certain you use the proper safety gear.
Gloves must be insulated to protect hands from freezing and must be impervious to anhydrous ammonia. In addition, the gloves should be cuffed to prevent liquid ammonia from running down the operators’ arms when they lift their hands.

Goggles can be non-vented or indirect vented. Safety shields do not take the place of goggles. In addition, employees who work around anhydrous ammonia should never wear contact lenses.

Golden Rule Number 2
Always check the water supply in the emergency soak tank that someone would jump in in case of an emergency.
Tanks collect algae, dead animals, and trash. Would you jump into a tank full of debris or snapping turtles? Remember that water is your best friend when you have been exposed to anhydrous ammonia. Water will relieve pain and minimize skin damage from ammonia exposure. However, if a catastrophic release occurs and getting to your emergency water source puts you in harm’s way, run up wind and find an alternative source of water outside the danger zone.

Golden Rule Number 3
Inspect hoses for cuts, nicks, or soft spots.
Hoses that come in contact with metal risers can result in the hoses becoming abraded over time. Small pin pricks are normal. Never take a chance with a damaged hose — replace it! Never use duct tape or black tape to repair a hose, not even for a short-term fix.
NEVER CUT CORNERS WITH ANHYDROUS AMMONIA
Every facility has a risk management plan that lists standard operating procedures (SOPs) for how ammonia should be handled at a facility.
SOPs can vary from company to company and even branch to branch within a company. To use a sports metaphor: the SOP is the playbook or game plan.
Company personnel should review their SOPs in the spring and again in the fall. The review will often cover such items as: how valves are labeled, how to open valves on storage tanks, how to fill nurse tanks, how to safely disconnect hoses, what safety equipment to wear, how to shut down systems in the event of emergencies, and how to transport nurse tanks on the highway.
An SOP is more than words on paper. It is a step-by-step process that describes how to work safely around anhydrous ammonia. Cutting corners and not following procedures puts any ammonia releases squarely on the shoulders of the employees who decided to circumvent those established procedures.
Follow the procedures so you can return home at night.
Components of an anhydrous hose:
Prevent Hose ’Basketing’
When stainless steel braided anhydrous ammonia hoses balloon, or mushroom out behind the coupling, it is called basketing. This occurs when anhydrous ammonia is left in the line, heats up in the sun, and expands. By design, manufacturers allow hoses to expand behind couplings so the hoses don’t burst. Prevent basketing by completely emptying hoses after each use. Some manufacturers evenly space pinpricks on hoses to help prevent gas buildup.
After your protective equipment is on, you filled the emergency water tank with water, and checked the hoses, it is useful to take a general walk around the riser before the day’s work begins.
Consider the following when filling tanks with anhydrous ammonia.
• Always assume ammonia is in the hose — just like you should always assume a gun is loaded! There is always ammonia residue left in the nurse tank that turns into vapor. Make sure to bleed hoses using valves designed to do so.
• Keep the platform clear of any trip hazards such as tools and hoses left on the deck. Many risers do not have guardrails for fall protection surrounding them.

• Fill nurse tanks no more than 85 percent using the fixed liquid level gauge as the main indicator.
• Be alert to potential problems. For instance, anhydrous ammonia fittings are designed to be hand tightened. If you are unable to hand-tighten the valves, it could be because the O-rings are damaged or the threads are worn. If you cannot hand-tighten the fittings, then you should stop and to see what the problem is. If something doesn’t appear right, stop and investigate. Attention to detail is critical. You should never use a pipe wrench to tighten fittings, because doing so can damage the threads.
• Keep unauthorized people off the risers. All ag retailers know that there are times when there is not a spare tank on the lot — everything is in the field. This means that when people are returning empty tanks, they want a quick turn-around on their fills. While it can be difficult, remember to keep people away from the risers as much as possible. The personnel filling tanks must focus on doing their jobs. Having two or three people on or around the platform can become distracting.
Designate a specified drop-off area where clients bring their empty nurse tanks. This will help keep everyone’s focus on what needs to be done.
One of the problems ag retailers face is having growers who bring in two tanks (one behind the other), drive across the scale, and then go directly to the filling station. Always tell growers why they can’t go up directly to the riser. We suggest they go to the office or another location on-site while you fill the nurse tanks. That way you don’t feel rushed or have to make idle talk with them that can take away from your concentration on the task at hand. More important, if an accident occurs, you don’t have to worry about the safety of the growers.
This might not be followed 100 percent of the time, but it should be the goal. There is no reason for growers to be within the area where tanks are being filled. Insurance companies sometimes require buffer zones for non-employees for certain operations as well.
MOVING NURSE TANKS TO AND FROM THE RISER
When application seasons begin, there is normally a person filling nurse tanks and shuttling empty tanks and full tanks around the lot. As the application season gets busier, other employees may be asked to move tanks.
There are a few safety considerations that apply to the person moving nurse tanks. Employees should always remember:
• Nurse tanks are bulky and heavy. Take care not to drop the nurse tank tongue on your feet or smash your fingers at the hitch.
• Individuals who move nurse tanks should wear the same PPE as employees on the riser at the filling station. Some companies require any employee within a 25-foot radius of the riser to wear gloves and goggles.
• Know where to activate the emergency shutoff in the event that the person on the riser is unable to do so.
• Every nurse tank should be chocked when parked at the riser. This prevents any forward or backward movement.

• When nurse tanks are being filled with anhydrous ammonia, they should not be attached to a vehicle in case someone tries to drive off.
Pull the hitch pin out and unhook the safety chains. Let the tongue sit on the drawbar for a quicker reattachment.
• Nurse tanks need a quick visual inspection. Pay attention to the condition of the tires; make sure the lugs appear tight; that the 5-gallon water tank is full, that there are no loose bolts; that placards are legible, not faded or folded; that there are no signs of leaks; and that the hitch pin, keeper, and chains are in good shape.
If something on the nurse tank needs repair, tell the manager or mechanic it needs attention. Put an out-of-service tag on the nurse tank to keep it from being put back into service until repairs are made.
Although anhydrous ammonia is exempt from certain regulations, it is still a hazardous material. From a retailer’s standpoint, a DOT out-of-service violation is a serious matter.


These photos show an ammonia release that resulted from a failed hitch. The pin came out and the hose was pulled apart. The soil around the tank was frozen, but was able to be excavated and then incorporated back into the same field.
Can you see any problems in each of the photos?
A. Faded SMV emblem
B. Hose needs replacing
C. Decal coming off
D. 1005 placard faded
Transporting Anhydrous Ammonia In and Out of the Facility
Most of the estimated 16,000 nurse tanks in Indiana belong to agricultural retailers. Whether farmers pick them up or retailers deliver them depends on the trade area. In some areas, 95 percent of the farmers pick up nurse tanks from retailers. At other facilities, 95 percent of the farmers pay to have them delivered to the fields of application. Approximately half of the equipment used to apply anhydrous ammonia is owned by retailers and is rented to their customers. Retailer-owned equipment that is rented to farmers puts retailers in the liability crosshairs.
All nurse tanks and application equipment receive a quick walk-around inspection. You don’t need to be a trained mechanic to see a missing lug nut, notice the cord through the tire tread, observe that placards are partially torn, see that the SMV emblem has faded, or discover that a 5-gallon water container hose has dry-rotted from sitting in the sun all year.

IF YOU OWN IT, YOU’RE RESPONSIBLE FOR IT
When retailers own the nurse tanks and applicators, equipment maintenance is their responsibility. Poor maintenance subjects companies and responsible employees to increased scrutiny, questioning, and liability.
Generally, maintenance on tanks and applicators happens in late winter or early spring, right before they are placed into service. The employee’s job during the application season is not to complete the full inspection that mechanics conducted during the off-season, but to watch out for immediate needs as the equipment experiences wear-and-tear.

These are the small things that need your attention before you place any nurse tank on the highway. If a grower has an incident while hooking up to a set of tanks, any problem from the maintenance side of things might entangle the retailer in a lawsuit.
There are a number of considerations that affect the transportation of nurse tanks. Always keep these points in mind before putting a nurse tank on the road.
Make sure the equipment is road-worthy. A nurse tank is only as safe as the condition of the structure (for example, running gear) it is attached to. A poorly maintained nurse tank structure compromises the integrity of the nurse tank itself. This is why it is just as important to inspect the nurse tank’s structure as it is to inspect the nurse tank itself.
Make sure the slow-moving emblem can be clearly seen and is not faded.
Make sure the 5-gallon water tank is full and the hose is not brittle or clogged.
Make sure that the farmer’s valve is completely closed and the dust cap is secured.
Make certain that the hitch pin, safety clip, and safety chains are securely hooked.
One safety tactic is to cross the chains to form an X pattern under the tongue. In the event the nurse tank becomes detached, the chains will serve to cradle the tongue above the roadway. Don’t forget that safety chains are not only hooked up between the truck and the nurse tank. You should also hook up safety chains between nurse tanks if you have a trailing nurse tank.

NOTE INDIVIDUALS WHO WON’T USE CHAINS
Some farmers will not use safety chains even after you have asked them to. Individuals will let you know that it is their decision to use them or to leave them off. They might even say, “Mind your own business. I’m not going to use safety chains. If it rolls over, I don’t want the nurse tank to take my truck with it.“
Imagine if, down the road, the tank rolls and hits an oncoming car. For that reason, every time a farmer refuses to put on chains, you should train your employees to note that on the order ticket in case this is called into question in a deposition or court.
Plan the travel route before you leave the facility.
Do not forget: You are transporting a hazardous material. Your goal should be to get the tank to the field of application and the empty tank back to be refilled as directly as possible. When pulling a nurse tank from a retailer location to a field of application and back, do not make any unnecessary stops. Don’t be tempted to stop at a drive-through, do business at the bank, or pick up supplies at the store.


Never transport anhydrous ammonia through town. It might be the quickest route, but it won’t be the safest. You have to think of the consequences of having a roadside incident with a nurse tank in a populated area. It’s true that there is not a law against going through town with a nurse tank unless you are informed that an alternative route has been designated for moving hazardous materials. It might take you an extra 5-10 minutes to take a route that does not include going through town, but if you do have an accident and ammonia release on the road, you won’t be in a highly populated area.
Observe the 25-mph speed limit. The speed limit for a slow-moving vehicle is 25 mph. There are good reasons for this.
First, speeds greater than 25 mph increases stopping distances, regardless of whether you are pulling one tank or two, filled tanks or empty tanks, or two tanks side-by-side on a single running gear. The more weight a vehicle pulls at faster speeds, the greater the distance it will take to stop.
Second, as speeds increase, nurse tanks begin swaying back and forth, which can cause the driver to lose control. At faster speeds, hitting a rough spot in the road will start the nurse tank weaving back and forth.

Third, driving too fast on speed-rated tires that are meant to only go 25 or 30 mph can lead to blowouts and overturned nurse tanks.
The fourth, and last reason, is that an SMV sign tells the public and law enforcement that you are moving at 25 mph or less.
Stop at all railroad crossings. Federal law requires equipment transporting placarded hazardous materials to stop at all railroad tracks.
Be prepared to deal with incidents that involve an anhydrous ammonia release. This means that you should always carry the gloves and goggles for anhydrous ammonia when transporting this material in case you can help mitigate the problem. No one plans for an incident on the road, but you should always plan to respond to one should the need arise.

Imagine how this would have ended if the anhydrous ammonia tank would have been hit.
Applying Anhydrous Ammonia
Farmers also are involved in transporting nurse tanks from ag retailer locations to their fields or between fields. Farmers who take possession of nurse tanks are involved in the additional step of applying the ammonia from nurse tanks into the soil.
In other words, those who make applications are actually doing a controlled release of anhydrous ammonia for plant nutrition.
There are a number of steps applicators must follow if the fertilizer is to be applied safely as a nutrient. These steps include:
Always Wear the Right PPE
Make sure to wear the right kinds of gloves and goggles when working on any aspect of anhydrous ammonia equipment. You should always put on the gloves and goggles before walking up to the nurse tank or applicator.


Assume ’Live’ Equipment
Always assume that anhydrous ammonia is in the hoses and that the nurse tanks and application equipment are pressurized.
Install the Coupler Correctly
The quick connector/breakaway coupler needs to be correctly installed if it is to pull apart and stop the flow of ammonia. The purpose of the L-brackets is to allow a straight pull. Without the straight pull, the coupler will bind, and then the hose will either get pulled apart or the valve will be ripped from the tank.



The breakaway coupler is mounted on double L-brackets and secured to a mounting bar. The mounting bar has a double-bolt system attached to the double L-brackets along with a bushing for the breakaway coupler.
The two L-bars swivel up and down and right to left, which keeps the hose in a straight line between the applicator and the nurse tank. If the tank separates from the applicator (for example, when a tongue or hitch pin breaks), the coupler should pull apart, causing the check valve to stop the flow.
It is critically important to follow manufacturer instructions for properly installing all breakaway couplers and to follow the recommended service periods. Most breakaway coupler failures are related to improper installation or lack of maintenance. This becomes an issue not only for farmers, but for retailers, too — some outlets will apply ammonia for 20-30 percent of their customers.
Can you tell which breakaway system is correctly mounted? Actually, both A and B are installed incorrectly. Photo A has two L-brackets that allow vertical and horizontal movement, but is missing the mounting bar. Photo B has only one L-bracket and is missing the mounting bar.
Inspect and Service Coupler on Both Ends
You need to inspect and service (that is, grease) the breakaway coupler on both ends. You also need to “exercise” the coupler in order to separate in the field of application. Inspection and service should be part of the preseason check before going to the field. When the breakaway coupler doesn’t disconnect easily and smoothly, the hose can be pulled apart or the farmer’s valve (liquid withdrawal valve) on the nurse tank can shear.
Always Use Bleeder Valves when Disconnecting Hoses
Take Care Standing on Tires
Be careful standing on tires, especially when it is wet. When you connect the hose from the applicator to the nurse tank, that usually means climbing on the front tire. Falling from the tire can cause more than personal injury — the hose may be damaged or broken, or the valve may be inadvertently opened.

Secure the Safety Chains
Ensure the safety chains are secured with a good pin and keeper. The chains, clips, and hitch are not only for the nurse tank but also for the applicator to the tractor. These connection points are critical for keeping individual units together.
Never Transport with Valve Open
Never transport a nurse tank on a public road with the farmer valve open and the hose connected to the applicator. If you are driving a tractor with the application tool bar plus nurse tank, disconnect the transfer hose and make sure all tank valves are tightly closed.
Shut off or close all valves at the tank source, which is especially important with double tanks on a single running gear. There are valves where the two hoses meet at the bulkhead along with primary valves on each tank (see red arrows in the photograph below). Shutting off„ the valves at the bulkhead does not shut off the primary valves at the tank. Failing to close the valves creates a live system. Whenever you tow or transport a nurse tank on the road (even behind an applicator) state and federal regulations require you to turn the valve off and disconnect the hose.


Farmers Should Mount Safety Water on Applicators
Farmers who own their application equipment should mount safety water containers on their applicators to make sure water is available in case of a release. Never assume there is water in the tank. It’s more common than you expect for the clear hose to pop out of the container, allowing the water to drain out.
The plastic of the clear hoses will degrade in the sun causing brittleness and breakage. For these reasons we also suggest that anyone who makes applications keep a small squeeze bottle of water in their pocket and a gallon container in the cab. There may not be time in an emergency to access the 5 gallons of water on the nurse tank.

Inspect Hoses
Check ammonia hoses for nicks, cuts, or soft spots before using them. Replace any damaged hoses before putting the unit back in service.
Check Hose Expiration Dates
Make sure all hoses are within their expiration dates. Replace any hoses that are beyond those dates.

Check O-ring Gasket
Make sure the O-ring gasket in the withdrawal valve is present and functional. While wearing your gloves, rub your fingers in the connector to make sure the gasket is there. Hand-tighten the hose end valve from the applicator to the nurse tank. Open the hose end valve all the way, then proceed by slowly opening the withdrawal valve on the nurse tank. This slow approach allows you to notice any leaks while the valve is slowly releasing product into the transfer hose.

Fully Open Withdrawal Valve
Make sure to open the withdrawal valve and hose end valve of the transfer hose to its fullest open position. If that valve is not opened all the way, it will impede the activation of the excess flow valve on the nurse tank.
HUMAN ERROR IS BEHIND MOST ANHYDROUS AMMONIA RELEASES
Retail employees and farmers who routinely handle anhydrous ammonia sometimes forget this fertilizer is an extremely hazardous material. During busy times of the year, retailers are handling several other fertilizer products, making sure that the bulk ammonia storage and nurse tanks are kept filled, and getting nurse tanks to the field safely. Some get lax in their anhydrous ammonia handling, because they have not experienced a release.
This brings up the question: “Does familiarity with anhydrous ammonia and fatigue from working long hours breed complacency and increase the problems we see when filling nurse tanks, transporting them on the road, and making the fertilizer application?”
This question is important to ask, because handling anhydrous ammonia at the retail facility, transporting nurse tanks on the highway, and making field applications are where accidental releases occur across the state every year. It doesn’t take long to understand the demands of the season and to forget about the dangers of anhydrous ammonia when the job becomes monotonous, and the pace quickens as more growers place their orders for this product.
We know from experience that fatigue can lead to complacency when users bypass standard operating procedures in order to get nurse tanks filled and transported out to customers. For example, a grower brings in a nurse tank to be refilled, and they are in a hurry to get back because heavy rains are predicted for the area. The retail employee bypasses chocking the nurse tank tires and disconnecting it from the towing vehicle, trying to get the farmer who is waiting back to his fields. The good news: nothing happened that day, and the farmer was satisfied to get in and out quickly.
This transport was beginning to offload into a facility storage tank. The operator failed to chock the wheels and set the parking brake. During the offload, the transport rolled backwards, which broke the piping on the transport and released more than 7,000 gallons of anhydrous ammonia. More than half the town had to be evacuated and more than 25 residents were treated at the local hospital.

But what could have happened, or what might happen in the future given the same set of circumstances? The photos on page 46 show what can happen when a driver fails to chock the tires of his cargo tank delivery truck. What was a simple procedure to follow was not followed, and the result was a release that caused an early morning nightmare for a community.
The bottom line is that it comes down to ag retail employees of who are dealing with customers who need the product quickly. Those employees need to follow safety procedures. Taking shortcuts during a short season can lead to errors when filling and transporting nurse tanks during spring and fall applications.


Human Error: An Avoidable Catastrophe
The driver of a tractor towing an applicator with two side-by-side ammonia tanks on the highway between fields found himself in a terrible predicament. One minute, everything was fine, but the next second he was involved in the release of 750 gallons of anhydrous ammonia in a populated area.
Eventually, the gas sent dozens of people to local hospitals. Among the injured were those working for fire departments and law enforcement. Authorities issued a one-mile shelter-in-place order. After attending to their patients, emergency room staff began complaining of ammonia exposure symptoms. The hospitals placed decontamination units outdoors to take care of the injured.
The tractor operator did not call 911 — another motorist placed the call. That was the biggest problem. Had the tractor operator called in the release, he would have stated it was anhydrous ammonia. The motorist who called in the release stated it was a car fire. Emergency responders were confused about the source of the “smoke” — many thought it was a car fire. Many of the early responders were seriously injured when they came into contact with the ammonia cloud.
Initial media reports blamed the applicator who had been towing two side-by-side tanks with a tractor on a public road. That in itself was not the problem, but pulling or towing ammonia tanks on a public road with the valves open and the hoses fully charged was the issue.
The applicator was unable to turn off the valves due to the cloud. If he had just followed the regulations and used common sense of not traveling between fields with the system charged, this incident would not have happened. Once again, this release was attributed to human error.

Consider these common factors at ag retailers:
• Retailers often limit which employees work full-time with anhydrous ammonia. However, during the spring and fall application seasons, inexperienced employees are often asked to assist.
• As the fertilizer season unfolds, employees work long hours each day and, at times, seven days a week. When there are rain delays, retailers face extra burdens when the weather clears enough for field applications.
• Filling nurse tanks and moving them is repetitious work. Employees on risers may be filling nurse tanks on one side, while disconnecting the hoses from the filled tanks on the other side. Meanwhile, people on the ground are connecting and disconnecting nurse tanks as one tank is filled and another is placed in line to be filled.
The redundancy of filling nurse tanks, transporting them to farms, making applications, and retrieving empty nurse tanks for refilling can become tedious. It is easy to become complacent and less focused when you work under these conditions. This can lead to accidents, such as driving away from the filling area with the hoses still connected, which could cause a release.
• Farmers recruit family and friends (who may not have any experience pulling or towing nurse tanks) to transport nurse tanks. The recruits sometimes do not understand how much distance they need to stop with the additional weight.

An untrained employee may hustle to get tanks to a farmer who has run out of anhydrous ammonia. This sort of “encouragement” may signal to the driver to do whatever it takes (such as, drive too fast or through town) to get it to the farmer who is waiting idly in the field.
Highway incidents are likely when drivers exceed the 25-mph speed restrictions for nurse tanks. A truck that weighs 8,000 to 11,000 pounds may transport two nurse tanks that weigh a total of 15,000 to 22,000 pounds. One problem with nurse tanks is that a high percentage are not equipped with brakes, because they are implements of husbandry. The truck must provide all of the breaking power for a heavy load. Such a heavy weight pushing behind a truck means it will take longer than anticipated to come to a complete stop.

When a nurse tank veers off the road and onto a shoulder at higher speeds, bad things happen. Most shoulders on rural roads drop quickly, resulting in the tank becoming unstable. Pulling back on the roadway often leads to losing control of the nurse tank, which could cause a rollover.
These photos depict nurse tank rollovers that occurred during transport. Don’t let this happen to you!
This rollover was caused by a combination of speed and missing tank bolts.
• Performing preventative maintenance during the off-season is critical, because equipment on nurse tanks can fail if they are defective, worn, or incorrectly installed. For example, using a high-pressure hose beyond its expiration date is trouble waiting to happen. Furthermore, bolts can break or welds can fail on nurse tank carriages (also known as the running gear).
THE GOAL: REDUCE HUMAN ERROR
Most anhydrous ammonia releases are directly linked to human error. It’s clear that reducing these mistakes is at the center of all training programs.
That’s not to say that all anhydrous ammonia releases are a user’s fault. In many documented cases, incidents are caused by those sharing the highway. Another driver may run a stop sign and crash into the nurse tank. This may rupture the nurse tank shell or shear o‚ff valves on top of the tank.
What was supposed to be a normal day turns into a crisis with a major release. Emergency responders try to stop the situation, ambulances evacuate the injured, and nearby residents must remain in their closed-up homes.

Preparing Before an Emergency Occurs
EMPLOYEE TRAINING
Can you remember the initial anhydrous ammonia training you went through when you started working?
It is often the case that new employees will watch a video or two about anhydrous ammonia. This is often followed by introducing employees to hands-on experiences by teaming them with people who have worked with the product for years. Working at the riser allows new employees to actually connect and disconnect hoses, learn the purposes and functions of the many valves, and how to properly connect and disconnect nurse tanks. This one-two approach to anhydrous ammonia handling safety training helps put the classroom information into real-world practice.
Can you remember the first time you were working on the riser or moving nurse tanks around?
During the first week, you were probably very cautious about how you worked around ammonia and followed the policies of the facility to the letter. Did you notice how comfortable you became with anhydrous ammonia after about one month? Gaining confidence from experience is a good thing, but getting cocky will lead to problems. Management wants employees to stick to safe practices and use common sense.
Most ag retailers offer basic anhydrous ammonia training to the entire staff — applicators, field scouts, office personnel, salespeople, and agronomists.
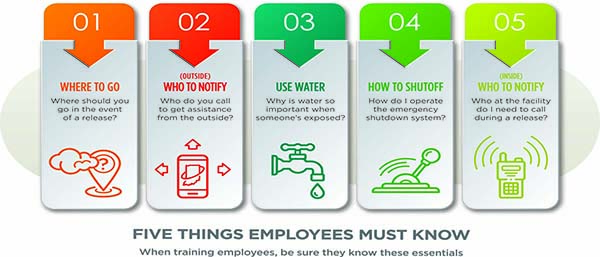
There are five points every facility employee must remember about anhydrous ammonia:
1. Where do you go when a major release occurs?
2. Who do you call to get outside assistance?
3. Why is water so important when someone is exposed?
4. How do I operate the emergency shut down system?
5. Who do I need to call at the facility when a release occurs?
If employees can answer these five questions, then the training session is deemed successful.
Why would employees who do not handle anhydrous ammonia need to know how to answer these five questions?
Your life and theirs may depend on them doing the right thing during an ammonia release. In addition, shutting down the system may avert a catastrophic release, keeping a minor release from becoming larger.
This basic training is part of management’s accountability to its employees and response teams. When emergency responders and the facility’s risk manager arrive at the scene, a quick head count is the first step in ensuring that all employees are present and accounted for. When someone is missing, rescue teams will risk their lives to find and rescue that missing person, which then endangers them.
Safety coordinators also can direct individuals at the meeting location to move to another location to allow emergency responders in and keep others out. In other cases, the employees can run errands or make additional phone calls when management asks. For instance, a manager may ask an employee to take a Safety Data Sheet (SDS) to the emergency room if it was not provided when the ambulance left the facility with an injured person.
RUN UPWIND IF YOU CAN’T GET TO EVACUATION POINT
In the event of a catastrophic anhydrous ammonia release, you may not always be able to reach the designated evacuation site.
When that happens, your goal is to save yourself by quickly running upwind from the release. The fact of the matter is that you are in survival mode at this point. When it is safe to do so, you should make it to the evacuation point or, at least, call management to let them know you are safe.
IRE DEPARTMENT TRAINING
Training volunteer and paid fire department personnel is important to ag retail facilities for two reasons:
1. Fire departments need the training, because anhydrous ammonia releases are something they seldom encounter.
2. Not all fire department volunteers come from agriculture backgrounds, so many are unfamiliar with anhydrous ammonia.
Training fire department personnel is critical to the well-being of everyone involved. This is especially true for urban fire departments that cover rural areas. As towns and cities encroach on farm ground, the chances of a roadway accident involving nurse tanks increase exponentially.
Fire department training varies depending on the need. The training can be as simple as the fire department touring the facility each year. Those on the tour can ask questions and familiarize themselves with the facility’s layout and operation. The walk-through also helps the fire department better understand what challenges they need to plan for in the event they are asked to provide
assistance. In some cases, ag retailer trainers conduct in-house training programs or conduct hands-on exercises to train local fire departments.
Providing the training to fire departments also builds understanding among ag retailers, farmers, and emergency responders. In the event of a release. That awareness becomes crucial when everyone must make important decisions.

Reacting During a Medical Crisis
Accidents happen, so what is your role if you are unlucky enough to be present at the time of an ammonia release?
The truth is there is not a playbook that lists the steps that one should follow to deal with an anhydrous ammonia release or even injured people. It becomes especially complicated to determine what to do when injuries and ongoing releases occur simultaneously!
While no publication, video, or even experience can tell you exactly what to do when handling medical emergencies that involve a person exposed to anhydrous ammonia, there are common eight steps you should take.
STEPS FOR DEALING WITH INJURIES
Whenever someone has been exposed to or injured by anhydrous ammonia, you should follow these eight steps.
Step 1 — Remain Calm
The situation is serious, and you have to convince injured people to follow your instructions. That will only happen if you remain calm.
Step 2 — Move Injured People Upwind
As soon as you’re able, move injured people away from the ammonia release by going upwind from the release. A wind sock at the filling station can be helpful in indicating which direction the wind is coming from.
KEEP FROZEN CLOTHING FROM RIPPING SKIN
People who have been injured may tell you their chests are on fire from the anhydrous ammonia on their shirts. Logically, the injured people want to pull their shirts off.
You must convince them not to remove their clothes until they get into the water.
The anhydrous ammonia might have frozen their clothing to their skin. Only take off clothing after they are in the water and the clothing has thawed. The good news is that the water will give exposed individuals almost instant relief and take their minds off removing their clothing. You just have to communicate to them by firmly saying, “Hang on, let’s get you to water as quickly as we can. Once you are in the water, the pain will be relieved, and then we can pull your clothing off.”
Putting exposed people in water helps in another way. Trying to remove frozen clothing might further injure the people, because doing so could rip the skin from their bodies. Make sure clothing is thoroughly saturated before very slowly proceeding to remove clothing.



Jumping into contaminated water can further aggravate skin injuries from ammonia exposure. Make sure your emergency water is always clean.
Step 3 — Provide Water, Water, and More Water
In just about every scenario, your need to make sure to take injured people to the facility’s water tank located upwind from the riser. Keep injured people in the tank until further help arrives. Water in a nearby ditch or creek may provide alternative sources of water.
Step 4 — Activate Emergency Shutdown System
With injured people in the water tank, it is time to quickly activate the emergency shutdown system — if you can do so safely. Even if you activate the shutdown system, you still must call 911 to get emergency responders to the facility.
Step 5 — Provide Only Life-saving First Aid
When someone is injured from ammonia exposure, the only things you should do are perform CPR if the person is not breathing or stop any bleeding. Never put any ointment or salve on burns, because that can cause the ammonia to travel deeper into the skin. When that happens, the ammonia will be trapped in the body and the water will not be as effective. Medical personnel will decide what the best treatment options are for any injured person. We are not doctors, so don’t pretend to be one.


Step 6 — Call 911
Employees who call 911 should never be faulted for calling 911 if, in their best judgement, help was needed.
An important step in the communication process is to inform 911 dispatchers that you are reporting an anhydrous ammonia release. Anhydrous ammonia is a placarded hazardous material, an inhalation hazard, and has a 1005 decal.
Dispatchers will also need to know if the release is ongoing, how many people are injured, and the severity of those injuries. The 911 dispatchers will ask additional questions that you will answer to the best of your abilities.
Step 7 — Answer Questions from Emergency Responders
Be ready to provide a safety data sheet (SDS) to any responders. Responders will debrief you about what you know and the actions you have taken. Later on, your safety manager will ask similar questions as he or she documents the facts while they are still fresh.
Step 8 — Send the SDS to the Emergency Room
Once emergency responders arrive, make sure to give them the SDS for anhydrous ammonia. The information on the SDS is important for emergency responders transferring injured people to emergency rooms. Just as important, the SDS also may help guide the responders on how to deal with the escaped vapor.

FOUR SCENARIOS
Now that you know these steps, consider these scenarios. How would you react in each one, using the eight steps as your guide?
Scenario 1
A person on the riser was exposed to anhydrous ammonia, but remains calm. What do you do?
Scenario 2
The employee on the riser tries to disconnect a hose by cracking it without bleeding it off„ first. One of his coworkers on the riser is not wearing goggles. They scream in pain as the vapor finds its way into their eyes. What do you do?
Scenario 3
The person working the nurse tanks sees his coworker on the riser fall on the deck, seemingly disorientated and confused. The wind is blowing the release from the ruptured hose away from the riser. What do you do?
Scenario 4
As you deliver full tanks to a field, you find a farmer lying near an empty tank blinded by anhydrous ammonia with burns on his skin. The release is ongoing, but the wind is carrying the ammonia cloud away from the scene. What do you do?
The two main differences among the four scenarios are the seriousness of the injuries and whether the releases stopped or are ongoing. The first question we should ask ourselves: Can the injured person be helped without much risk to the person offering assistance? Even so, it is important to remember that while we often categorize anhydrous ammonia injuries as “minor” or “serious,” the reality is that they are all serious injuries. In spite of what injured people say or think, you should treat any exposure to anhydrous ammonia as an emergency.
People injured from ammonia exposure often say, “Don’t call 911. I’m OK.” It really doesn’t matter what injured people say. A physician should evaluate all ammonia exposure injuries, no matter the perceived severity. Anhydrous ammonia is always going to seek moisture, and in doing so, it will move deeper into the skin unnoticed. What injured people feel now is not really an issue, but later on it could turn into a critical health problem.
ALWAYS SEEK MEDICAL ATTENTION
Every injured person, no matter how minor the injury appears, should seek immediate medical attention.
Most injured people will say that everything is alright, so it’s unnecessary to call 911 or go to a doctor. It is important to remember that ammonia can produce both acute (right now) and chronic (later on) effects.
An exposed person could spend the rest of the day feeling fine, but after taking a shower in the evening and laying down, that person may begin to experience symptoms. The exposed person may feel almost like he or she is having pneumonia-like symptoms — wheezing and gurgling in the lungs every time he or she breathes in and exhales out.
These symptoms are caused by the ammonia reacting with water in the lungs. This is why every injured person must be seen by medical personnel.
PLAY IT SAFE
You will have to make quick decisions based on the circumstances you find yourself in. You are now dealing with an injured person and an ongoing release that could bring you in contact with the deadly gas. If you are uncomfortable dealing with the injured person within a cloud, focus your attention on calling 911. The fire department will have self-contained breathing apparatuses that will enable them to deal with the release safely in the event they need to enter the vapor.
It is also true that you will be severely limited on what you can do if you turn over a nurse tank. If you don’t have gloves and goggles with you, then you will not be able to do anything even if a person is down in the cloud. With appropriate PPE and the wind blowing away from you, then you might be able to shut off the release valve.
Our first reaction is to help injured coworkers by shutting the valve off and stopping the release. It seems unacceptable to be told you cannot help. The reality is that by trying to save them, you could become another victim. All you have to do is think about the stories you’ve heard about attempted rescues in a manure pit, in a grain bin, or downed power line that end up with others losing their lives in the process of trying to save a family member or coworker.
You don’t want to end up being one of the victims, and if you get hurt, it will only delay emergency aid to them. A situation that was bad to start has now gotten worse. We all have to rely on emergency responders to perform the rescue when we are unable to stop the flow of anhydrous ammonia. We hope that you never find yourself having to make this difficult decision.
But don’t just take our word for it. This is a true account that could have had a much different ending with the hero becoming the victim.
A hose ruptured on the riser and the pump was running. The employee on the riser was able to escape. He pulled on the emergency cable but the system failed, and the entire facility was engulfed in ammonia vapor.
All but one person evacuated the facility as they were supposed to. The only person who didn’t get out was the driver of a transport cargo tanker that was unloading at the
bulk storage tank. When the wind shifted, the driver ran in and shut off„ the valve. He was not injured and was able to keep the release from getting worse.
While the outcome was good, it was probably not what we wanted to happen. While the act was noble, did the end justify the means? Luck was on the driver’s side that day. It goes without saying that calling 911 should be your first move. Do not die becoming a hero.
All but one person evacuated the facility as they were supposed to. The only person who didn’t get out was the driver of a transport cargo tanker that was unloading at the
bulk storage tank. When the wind shifted, the driver ran in and shut off„ the valve. He was not injured and was able to keep the release from getting worse.
While the outcome was good, it was probably not what we wanted to happen. While the act was noble, did the end justify the means? Luck was on the driver’s side that day. It goes without saying that calling 911 should be your first move. Do not die becoming a hero.

Responding to the Vapor Cloud
Major releases are those that leave the boundaries of ag retailer facilities or result from ruptured nurse tanks on the roadway. People in the surrounding communities may need to take protective measures to keep them safe until the release is controlled and mitigated.
When you call 911, the dispatcher relies on your description of the incident when they notify fire departments, law enforcement, and emergency responders closest to the release site. It is typically up to the fire departments to decide the best course of action to take to mitigate a release. While the fire department is heading out, they are formulating a plan of attack based on the information you have initially provided to the 911 dispatcher.
The questions the firefighters are trying to answer on their way to the anhydrous ammonia release site include:

• Are there injuries?
• How much ammonia was released?
• What is the source of the release?
• What direction is the wind blowing?
• Is the release moving upward, or is it hanging low to the ground?
• What is downwind of the release?
• How will I notify people downwind?
• Will the release require evacuation or shelter-in-place?
• Which roads will need to be closed?
• From what direction should we approach the scene?
• Can the release be controlled?
• Are we equipped to safely shut down the release?
• Is it a “runaway” tank with no way of shutting or slowing down the release?
• Will I need assistance from other fire departments?
• Will we need to call in a hazardous material team?

As the fire department is traveling to the site, they are receiving updated information. While they establish their plan, they fully understand that conditions on the ground could change, which could alter what they do next.
Anhydrous ammonia releases grow more complex as the gas mixes in the atmosphere and moves with the prevailing wind. Incident commanders at the scene will have to decide whether to put water over the cloud or to let the cloud dissipate into the atmosphere.
Ammonia picks up moisture from the atmosphere, which causes the dense white cloud. Initially, ammonia is lighter than air, so it ascends into the atmosphere. However, when anhydrous ammonia is released into air that is saturated with moisture (think of a typical, Midwest humid day), the cloud becomes heavier so it lingers close to the ground.

A FALSE SENSE OF SAFETY
Laws require every ag retail facility that distributes anhydrous ammonia to have two full-faced gas masks with ammonia canisters on standby. They also must have rubber rain suits, rubber boots, goggles, and gloves stored with the gas masks.
WARNING! If you have ever worn a respirator, you know they need to be individually fitted. How can you guarantee that these masks will fit tight around your face? The answer is: you can’t.
These gas masks are for escape purposes only.
They are not for performing rescues. Running into a cloud wearing these emergency masks can have serious repercussions. Running into an ammonia cloud is analogous to a person jumping into a manure pit or a grain bin to help someone overcome with gas or engulfed in grain. Often, people who offer assistance become victims themselves. Depending on the gas mask, it is a matter of seconds (not minutes) before you can be overcome by ammonia vapor.
The main purpose for these gas masks is to help people get out during an evacuation. The air-supplied respirators that the firefighters wear are designed to allow them to go into the release and attempt to deal with the problem head-on.
THE DECISION TO DO NOTHING
Sometimes, emergency responders determine that the safest course of action is to do nothing, as this account relates.
“We had an ammonia release from a nurse tank that happened on a rural road between two towns. The emergency responders shut the road down, and let the cloud dissipate into the atmosphere. The wind was keeping it away from any potential threat to people.”

ON-THE-SPOT DECISIONS ARE PART OF THE JOB
Emergency responders have to quickly assess the situation and make critical, life-and-death decisions. Here is one account as remembered by one emergency responder.
“I had a release right in front of the schoolhouse at 8 in the morning. A valve had been sheared from the top of the tank. Some children were already at the school, and more children were arriving. That was a tough day for me. You don’t want your actions and decisions to hurt people.
“Fortunately, the ammonia cloud went up into the atmosphere and went over the school. We had the children shelter in place, closed the school, and basically locked them in. We turned back all of the cars bringing children to the school. We couldn’t take the chance with the ammonia cloud coming into contact with human life.”
Questions
Should the applicator have applied anhydrous ammonia within such close proximity of the school?
Could that field have waited until a late afternoon or weekend?
Incident commanders must determine the path of the moving cloud and how much ammonia is yet to be released. Gathering all of the facts dictates what actions they take to mitigate the release. It goes without saying that taking care of injured people is their top priority.

Emergency actions will vary depending on the specific circumstances of the incident. For instance, the fire department may arrive at the scene to find a ruptured nurse tank in the middle of 100 acres of farmland. The vapor cloud is going straight up into the atmosphere. They will likely decide to let the cloud dissipate into the atmosphere, because it poses no threat to those living in the area. From the fire department’s perspective, this is a safer approach that protects the public and reduces exposing members of the fire department to the ammonia.
If a house is downwind of an ammonia release or the release is moving toward a town, the fire department has little choice but to put water on the cloud. Furthermore, they must immediately inform those living and working in the cloud’s projected path to shelter in place, or in extreme cases, evacuate.
Putting water over the cloud has a negative consequence: anhydrous ammonia and water form aqua ammonia. Aqua ammonia that enters waterways can be toxic to aquatic life. Most fire departments understand this dilemma, but human life takes precedence over adverse environmental impacts. It is up to facilities to activate their emergency response plan to get bulldozers and backhoes working to prevent the aqua ammoniafrom entering waterways.
DO VALVES FUNCTION DIFFERENTLY ON OVERTURNED TANKS?
Look at the photo. If the nurse tank is upside-down, does that change how the valves operate? Everything we communicate with our emergency signs, the colors of the valves, and all of the training indicate that orange is for liquid and yellow is vapor.
But what happens when the tank is overturned?
The farmer’s valve is now in the vapor space and the vapor valves are in the liquid space. This might be something important you should mention to emergency responders who may be unaware of this fact.
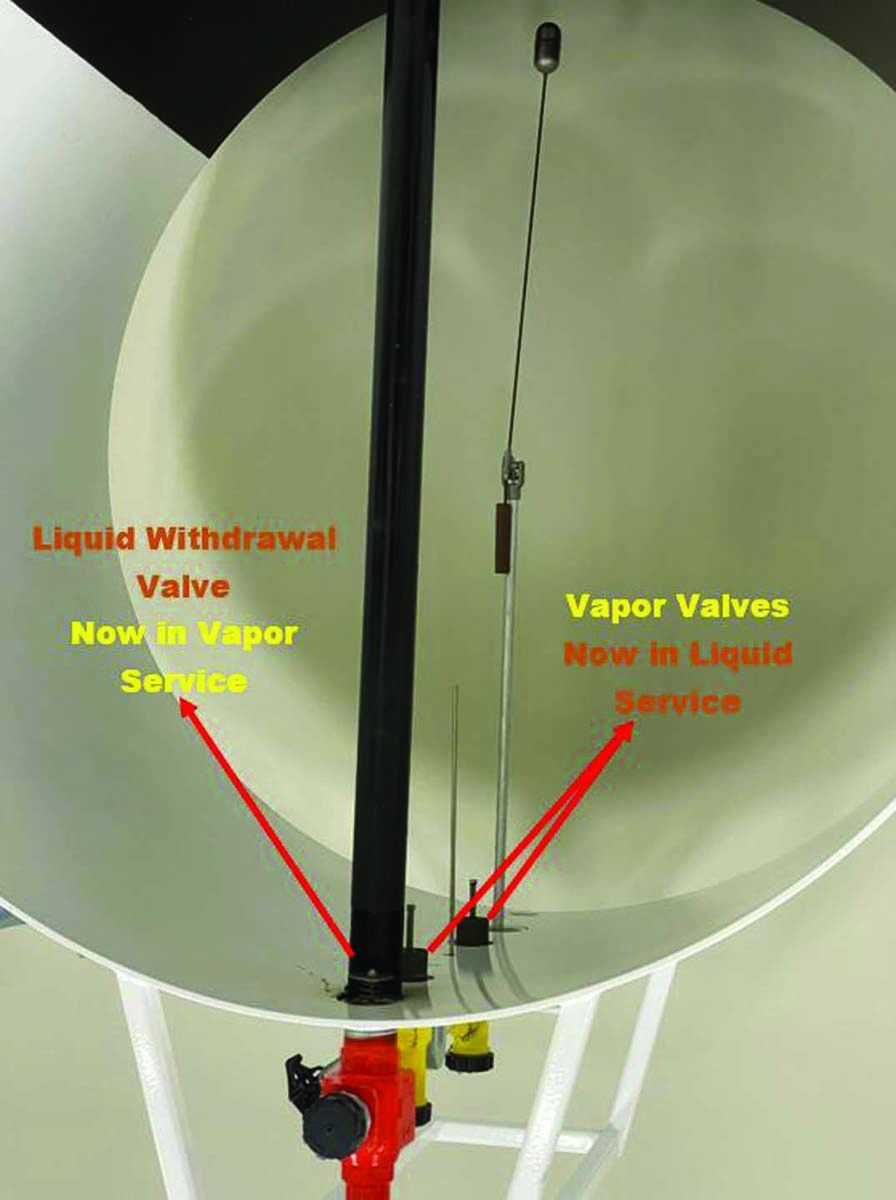
You might ask what this has to do with you.
If you are the one who called 911, then you will be expected to provide what you know of the circumstances surrounding the release. You will also be expected to repeat what you told the 911 dispatcher when the fire department shows up and to bring them up to speed about what has occurred since the call.
We hope that you now can appreciate what it means to stay out of populated areas. If you are the one pulling anhydrous ammonia tanks, do you really want to pull a placarded hazardous material through town? Pick routes that stay clear of populated areas, and don’t go through the bank or fast-food drive-through. It may not always be possible to avoid certain areas, but your goal is to always do so when alternative routes are available.
Mandatory Reporting of Anhydrous Ammonia Releases
Federal and state regulations require you to immediately report anhydrous ammonia releases. The reporting threshold for anhydrous ammonia is when more than 18 gallons or 100 pounds are released into the atmosphere.
In all, you must notify three agencies when an ammonia release meets the threshold: the National Response Center, the Indiana Department of Environmental Management, and Local Emergency Planning Committee. Calling 911 is not mandatory, but it is included in the list of first contacts to call.
You are required to call the National Response Center within 15 minutes of the release. Obviously, if people are hurt, you are not going to call before you treat the people and move them to a safe area. Taking care of the injured is a higher priority than making the required phone calls. It’s understood that the information may change as more information is collected, but the important part is calling 911 first, and then the other three agencies soon after.
Make the Call
National Response Center 800-424-8802
Indiana Department of Environment Management 888-233-7745
Indiana Local Emergency Planning Committee Contact List www.in.gov/dhs/
Always make the phone calls even if you are not sure that the release is above the 100-pound reportable threshold. There is no penalty if you call and authorities later determine that the release was below the reporting threshold. Penalties only come into play when calls are not made within 15 minutes.
Calling the agencies assumes that the threshold was exceeded. You can easily quantify the amount released by comparing the tank’s weight before and after the release. We know what the overturned tank initially weighed at the facility before sending it out. The damaged tank can be reweighed to determine how much anhydrous ammonia remains. Subtract the tank’s weight after the release from the tank’s initial weight, and that will give you the amount of anhydrous ammonia released.
Who is responsible for reporting releases that exceed threshold amounts?
Whoever is in possession of the tank is responsible for notifying the agencies. This means that a grower, who un-fortunately finds the retailer’s nurse tank leaking anhydrous ammonia on the roadway or in the field, is the one required to call.
Often, farmers first call the ag retailer, believing they are responsible for making the calls. However, because they were not in possession of the nurse tank, the retailer cannot make the calls, but they can provide the phone numbers to call. If the grower is incapacitated, the retailer will more than likely make the necessary calls.

How to Calculate Weights
3-ton Nurse Tank A ton is 2,000 pounds: 3 x 2,000 = 6,000 pounds (weight the tank is able to hold) The tank is filled to 85 percent capacity: 6,000 pounds x 0.85 = 5,100 pounds (weight in a filled tank) Anhydrous ammonia weighs about 5 pounds per gallon: 5,100 pounds ÷ 5 pounds/gallon = 1,020 gallons (in a filled tank)
2-ton Nurse Tank 2 x 2,000 = 4,000 pounds 4,000 x 0.85 = 3,400 pounds 3,400 ÷ 5 pounds = 680 gallons H
Conclusions
Ag retailers carry gasoline, diesel, pesticides, and fertilizers that they use or sell to farmers. Any spill or release of these products are important to the retailers and local communities where the facilities are located. This is why so much effort is required to train employees on how to prevent accidents and to educate fire departments on how to properly respond to them.
Of all the products stored at the retailer’s facility, anhydrous ammonia is of greatest concern. This is because releasing this hazardous material can seriously injure people, or even kill them. This is why it is imperative that everyone handling anhydrous ammonia follow the policies established by their facilities to the best of their abilities.
Much of the training for anhydrous ammonia aims to reduce human error and lower the likelihood that a release will occur. When we cut corners, don’t focus on the task at hand, or make a mistake, the result can be devastating not only for the facility’s employees, but to the surrounding communities.
Yes, it’s true that employees receive the same information each year at training. With anhydrous ammonia, nothing really changes, so the information doesn’t need to change either. But it’s important to remember that we are trying to make how we handle ammonia so ingrained that employees know exactly what they need to do when they are handling this important, but dangerous, fertilizer.
Unfortunately, what also doesn’t change from year to year are mistakes that result in anhydrous ammonia releases. Retailers spend time and money on employee training, plumbing maintenance, and equipment repairs. But the money they spend on training and maintenance is of little value when someone decides to circumvent what they have been taught.
The bottom line is to respect, but not fear, anhydrous ammonia. A brand-new employee who lives in fear of anhydrous ammonia is something we don’t want. On the other hand, we don’t want a seasoned employee to think, “I have handled it all of these years and never had a problem. I just don’t need to worry about it.” We don’t want you to be afraid of it, but we don’t want you to be complacent.
There are only a few, simple steps and guidelines you need to follow when working with anhydrous ammonia. Handled correctly, anhydrous ammonia is an effective and valuable fertilizer. Be informed, be safe, and put into practice what you have been taught.


A Passing Thought
Training is important. Here are the thoughts of one safety trainer.
“A safety coordinator can put things in place, but it is the employees who are my eyes on the ground. It is unfortunate that these types of releases occur. We don’t make them up. There are some who think that we stay awake at night trying to invent these stories as a way of screwing up their day. Events like this are real, they happen. And so, an employee has to speak up when something is not right. Working together allows us to prevent the releases.
Acknowledgments
We wish to thank the Office of Indiana State Chemist and the Cooperative Risk Coordinators Group for their financial support. Thanks to Dawn Minns for graphic design. Thanks also to the following individuals who provided valuable comments and suggestions that improved this publication.
Keith Fricke, Prairieland FS
Beau Gentry, Harbrand
Mel Mertel, Illinois Department of Agriculture
John Nowatzki, North Dakota State University
Jean Payne, Illinois Fertilizer and Chemical Association Mathew Pearson, Office of Indiana State Chemist
Shane Pruett, Illinois Department of Agriculture
Jason Solberg, Illinois Fertilizer and Chemical Association Dustin Warder, Asmark Institute
Disclaimer
This publication is intended for educational purposes only. The authors’ views have not been approved by any government agency, business, or individual and cannot be construed as representing a perspective other than that of the authors. The publication is distributed with the understanding that the authors are not rendering legal or other professional advice to the reader, and that the information contained herein should not be regarded or relied upon as a substitute for professional consultation. The use of information contained herein constitutes an agreement to hold the authors, companies or reviewers harmless for liability, damage, or expense incurred as a result of reference to or reliance upon the information provided. Mention of a proprietary product or service does not constitute an endorsement by the authors or their employers. Descriptions of specific situations are included only as hypothetical case studies to assist reader of this publication, and are not intended to represent any actual person, business entity or situation. Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind by Purdue University. Individuals using such products assume responsibility that the product is used in a way intended by the manufacturer and misuse is neither endorsed nor condoned by the authors nor the manufacturer.

Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind by Purdue Extension. Individuals using such products assume responsibility for their use in accordance with current directions of the manufacturer.
Find out more at:
The Education Store edustore.purdue.edu
June 2021