The Complex Life Of The Honey Bee

Fred Whitford, Director, Purdue Pesticide Programs
Thomas Steeger, Senior Science Advisor, Environmental Protection Agency
Max Feken, Ecotoxicologist, Syngenta Crop Protection
Christian Krupke, Extension Field Crops Specialist, Purdue University Entomology
Greg Hunt, Extension Honey Bee Specialist, Purdue University Entomology
Reed Johnson, Assistant Professor of Entomology, The Ohio State University
Corey Gerber, Director, Purdue Crop Diagnostic Training and Research Center
John Obermeyer, Integrated Pest Management Specialist, Purdue University Entomology
Krispn Given, Apiculture Specialist, Purdue University Entomology
A Complicated Question with a Complicated Answer ……………………………..4
The Buzz Surrounding Bees ………………………………………………………………….7
A Marvel of Nature …………………………………………………………………………….10
Building and Maintaining a City of Wax ……………………………………………….24
The Biology of the Honey Bee ……………………………………………………………..28
Death is a Way of Life ………………………………………………………………………..38
The Four P’s that Affect Bees ……………………………………………………………..40
Routes of Pesticide Exposure from Field to Hive ………………………………….42
Everything is Poisonous at Some Dose ……………………………………………….45
The EPA Risk Assessment Process …………………………………………………….46
The Honey Bee as a Surrogate for Insect Pollinators ……………………………48
A Host of Questions to Answer …………………………………………………………..49
Determining Many Routes of Exposure ………………………………………………52
Formulating a Working Hypothesis ……………………………………………………53
Analyzing the Data ……………………………………………………………………………53
Comparing Exposure and Effects in a Tiered Approach ………………………..54
Tier 1 — Effects to Individual Honey Bees …………………………………………..56
Tiers 2 and 3 — Colony-level Assessments ………………………………………….59
Is the Risk Quotient Above or Below Levels of Concern? ………………………61
Registration Decisions Go Through Extensive Reviews ………………………..62
The Label Puts the Risk Assessment to the Test …………………………………..63
Moving Beyond the Label ………………………………………………………………….64
Conclusion ………………………………………………………………………………………65
References ……………………………………………………………………………………….68
Appendix: Descriptions of EPA Testing Protocols ………………………………..69
Acknowledgements …………………………………………………………………………..75
Disclaimer ……………………………………………………………………………………….75
A COMPLICATED QUESTION WITH A COMPLICATED ANSWER
Are honey bees in decline?

Whether bees are healthy or unhealthy. You’ll often hear phrases like “bee decline” and “colony collapse disorder.” In many cases, these explicit terms become the headline, because they portray powerful mental images to the audience.
You might ask yourself, “How would I define these terms?” And, if a definition could be agreed upon, “How would I measure them?” Without some context to their meaning, bee decline, colony collapse disorder, and other terms can be rendered meaningless and confuse the issue.
Scientists, government officials, conservation groups, and industry professionals are asking plenty of questions about why beekeepers (both commercial and hobbyists) are seeing increased colony losses. One baseline often reported is that in a typical year, 28 percent of honey bee hives die during the winter season. Annual losses may be higher; this number is a source of considerable debate.
Whatever the annual baseline loss may be, the percentage of yearly losses began to routinely exceed that long-term average about a decade ago. When that happened, experienced beekeepers took notice, and many have voiced their concerns about the viability of beekeeping.
Any threat to beekeeping represents a threat to agriculture, specifically the production of fresh fruits, nuts and vegetables. This has raised the profile of the “honey bee decline” story and captured the public’s attention. We will discuss the potential causes for these trends in this publication, but regardless of the causes (at least in the short-term) these losses represent an unwelcome challenge for beekeepers. Left unchecked, these causes could potentially lead to more serious and widespread negative consequences.
Everyone from beekeepers to conservationists who want to protect diverse habitats want “The Answer” for the cause of bee declines to be proven using the best available science. While individuals search for a single cause, scientists continue to assert that, as with many biological questions, there are multiple factors associated with declines in pollinator health. The more we learn, the more the complexities become apparent.
Even so, this does not mean there are no solutions. We already know a lot about the threats to pollinators and pollinator research is more active than ever. For example, we know that the ectoparasite mite Varroa destructor arrived in the United States in the 1980s and is the single most devastating enemy of both managed and feral bees in the United States.
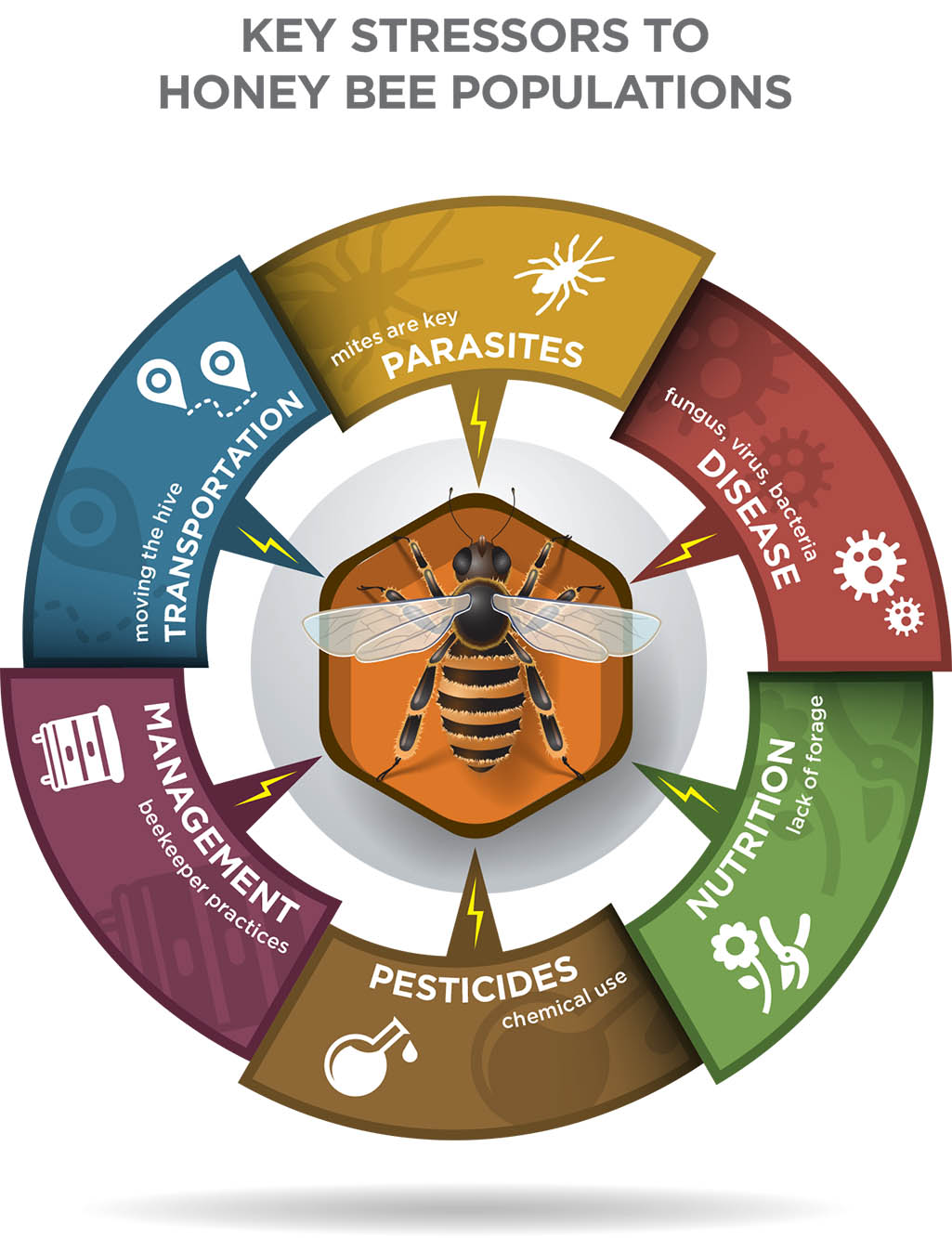
Other key stressors to honey bee populations
include:
• Parasites like tracheal mites (Acarapis woodi), small hive beetle, and wax moths
• Diseases (such as the fungus Nosema ceranae), bacteria (such as American foul brood), and viruses (such as deformed wing virus)
• Lack of suitable forage (poor nutrition) from loss of habitat due to farming, increased human populations, and new construction
• Pesticide exposure to honey bee foragers and their developing young (brood)
• Beekeeper management practices, which include the stress of transporting honey bees across the country for pollination services
While there will always be disagreements about the causes and effects of declines in hives, beekeepers, agriculturists, scientists, conservationists, and policymakers all agree that honey bees play a pivotal role in crop production, availability, prices, food security, and the economy.
A critical first step in protecting that role in the future is to outline the current state of knowledge surrounding the debate.
There are multiple factors associated with declines in honey bee health and outright colony losses. But the most controversial is pesticides, because this risk factor involves a wide range of stakeholders who agree that both pesticides and pollinators are important to agriculture.
This publication explores the roles pesticides may play in bee health, examines the latest knowledge in this fast-developing field of research, and discusses the regulatory effort to determine the risk of pesticides to honey bees and other pollinators. To interpret the role stressors like pesticides may play in influencing bee biology and health, it is important to understand bee biology. So, we will begin by examining that biology.
THE BUZZ SURROUNDING BEES
Though small, the honey bee (Apis mellifera) has become the center of an intense national and international debate. Starting in the early 1980s, commercial beekeepers noticed that it was increasingly difficult to keep honey bees alive through the winter. A worried commercial beekeeping industry asked university and USDA bee specialists and researchers to address why this was occurring.
Below, we summarize the recent history behind some of the key factors — tracheal mites, Varroa mites, and colony collapse disorder — known to affect honey bee populations in North America.
Tracheal Mites
One of the initial key contributors to declining bee populations was the tracheal mite (Acarapsis woodi), which was introduced from Mexico into a Texas apiary.
From that first detection in Texas, the tracheal mite quickly spread throughout the United States.
The microscopic tracheal mite enters a honey bee’s respiratory system through its spiracles, which are breathing holes that lead directly to the bee’s tracheae. Tracheae serve as breathing tubes, carrying oxygen to a honey bee’s cells. When tracheal mites infest a bee, they effectively clog these tubes, reduce oxygen flow, and weaken the bee.
Losses from tracheal mites forced some apiaries out of business. Researchers found that when 30 percent of the honey bees in a hive are infested with tracheal mites, the hive was more susceptible to cold winter temperatures. This was largely due to bees not maintaining a warm enough temperature within the hive cluster. Some apiaries reported that half of their hives died over the winter following the introduction of tracheal mites.
If there was a silver lining to the story, it was that honey bees and beekeepers developed various strategies to reduce tracheal mite infestations. Although tracheal mites continue to harm some colonies, the problem has mostly subsided into the background of honey bee health issues. For the beekeepers who remained after the initial tracheal mite invasion, they observed that the honey bee industry slowly began to recover.

VARROA MITES
Varroa Mites
However, that good news was soon supplanted when a new and far more serious threat was introduced. The aggressive Varroa mite was inadvertently introduced to U.S. honey bees around 1987. Domestic honey bees that had no previous association with this mite were extremely susceptible. As a result, apiaries recovering from tracheal mite infestation were decimated by the Varroa mite.
Varroa mites are an external parasite that feed on the hemolymph (insect blood). It feeds off adult honey bees and their developing young (larvae and pupae). Varroa mites also transmit deadly viruses — like mosquitoes can vector viruses to humans and other mammals. Mite infestations can kill pupae outright, and many of the adult bees that survive to emerge from pupal cells show symptoms of deformed wing virus, which can be vectored by the mites.
Deformed wing virus can render the bee wings crinkled and undeveloped. Bees infected with this virus cannot fly, so they cannot function properly as part of the colony. The virus weakens the bee and eventually kills it.
Varroa infestation in the hive typically peaks in the fall. Between the damage caused by the direct feeding of mites and damage caused by deformed wing virus (and other viruses), the hive loses a significant amount of its adult labor force. These losses make it more difficult for the bees to overwinter.
Varroa infestation in the hive typically peaks in the fall. Between the damage caused by the direct feeding of mites and damage caused by deformed wing virus (and other viruses), the hive loses a significant amount of its adult labor force. These losses make it more difficult for the bees to overwinter.
This is particularly unfortunate for the colony because a queen bee stops laying eggs in the fall, and the hive relies on heathy bees that can live through the winter to raise brood in the spring. Although it has been decades since the Varroa have been introduced, honey bees have not been able to overcome its devastating effects as they were able to with tracheal mites.
Throughout the 1990s, apiaries continued to make honey and provide pollination services despite the challenges presented by mite infestations. Beekeepers were forced to replace lost hives each year by splitting and placing healthy colonies into vacated hives, which stressed colonies even more. The result was a rapidly rising cost in agriculture.
Colony Collapse Disorder
In 2006 and 2007, a phenomenon known as Colony Collapse Disorder (CCD) made headline news across the country. CCD was initially defined based on symptoms: adult bees declined and the colony collapsed over the course of a few weeks, usually leaving the queen and a small number of workers in a colony with nectar, pollen, and brood still in the cells. CCD was never clearly defined, but the spectacular disappearance of bees gripped the public’s imagination in a way that continues today, even though the CCD syndrome may not have been as widespread or as long-lasting as media reports suggested.
Growers of fruits, nuts, and vegetables who depended on commercial beekeepers for pollination services began to worry if there would be enough honey bees for pollination. What had historically been taken for granted, took on a different meaning when the number of hives available for pollination services were significantly reduced (and therefore more expensive to rent).
In particular, almond growers provide the most compelling example of how much one agricultural crop relies on honey bee pollination services. California’s Central Valley grows approximately 80 percent of the world’s almonds. California almond growers rely exclusively on honey bees for crop pollination, which requires more than 1.8 million hives, or about two hives per acre.
More than half of all of the managed honey bee colonies in the United States are required to pollinatealmonds every single year. The cost for commercial pollination is about $300 million or about 10 percent of the total production costs for almonds. The cost to rent a single hive has increased from $15 to $30 to around $200 because the availability of colonies has become limited, the costs of maintaining the colonies has increased, and California growers have increased almond production. Ultimately, all of these factors are reflected in higher almond prices for consumers.
This is just a single example — there are dozens more with relevance for crops all over the world.
But there is more to honey bees than the services they provide, and understanding their remarkable biology is important to answer the question we posed at the beginning (Are honey bees in decline?). In the sections that follow, we will explore the amazing and complex biology of this insect before discussing how some of the stressors (including pesticides) affect them.

A MARVEL OF NATURE
Human civilization and honey bees have coexisted for thousands of years. As a direct benefit, honey bees provide us with products such as honey (a natural sweetener) and wax (for making candles and other products). Honey remains one of nature’s best and most versatile examples of a naturally produced product used in cooking, beverages, cosmetics, and medicines. Annual U.S. honey sales amount to $320 million. Americans consume 450 million pounds of honey each year, more than half of that honey is imported.
While honey is an important bee product, the greatest value of managed bees is in the pollination of nut, fruit, and vegetable crops. For example, commercial beekeepers transport tens of thousands of hives on semi-trucks to farms growing crops that require insect pollination. Bee managers transport hives to pollinate almonds in California; blueberries in Maine, Georgia, and Florida; cherries in Michigan; apples in Washington and New York; melons in Indiana; cranberries in Wisconsin; and sunflowers in North Dakota.
Some plants are self-pollinated (this includes most grains or oilseed crops, tomatoes, peppers, peas, peanuts, and citrus), and other plants are wind-pollinated (including corn, soybeans, walnuts, and pecans). But many flowering plants require insects to move pollen from male to female parts to produce a fruit, vegetable, or nut. A large percentage of the most colorful food items in the produce section of grocery stores must be pollinated by honey bees and other pollinators.
We are now learning that pollination by wild bee species also contributes
substantially to yields in some fruits and vegetables, including many types of melons, stone fruits, and berries. Bumble bees (Bombus species) are particularly well-suited for pollinating plants such as blueberries and tomatoes. Alkali bees (Nomia melanderi) and alfalfa leafcutter bees (Megachile rotundata) are important for pollinating alfalfa grown for seed.
Worldwide, there are seven honey bee species, but Apis mellifera is the species hobbyists and commercial beekeepers use most often. The native range of A. mellifera is in Europe, and there are different geographical races named after their respective areas of origin. These include the Italian, German Black, Caucasian, and Carniolan, many of which have become popular among beekeepers in the United States because of their specific rearing traits.
European settlers introduced the German black honey bee (Apis mellifera mellifera) to the United States in 1638. This specific race was well-adapted to cold temperatures.
In the 1850s, beekeepers imported the Italian honey bee (Apis mellifera liqustica), because it produced larger colonies, made more honey, was gentler and easier to manage.
Today, many apiaries typically include hybrids of these and other races. Although queen bees are sometimes artificially inseminated, the vast majority of queens mate while flying with multiple males (drones), which results in diverse genetic mixtures and genetic diversity in the hive. These genetic mixtures are hybrids that have different characteristics including disease resistance, foraging efficiency, aggressiveness, adaptability to fluctuating food availability, honey production, and cold-weather tolerance.
Scientists have long been fascinated with honey bees. The study of honey bee biology goes back as far as Aristotle (384-322 B.C.), making it one of the oldest subjects of scientific inquiry. And the economic importance of honey bees for pollination and food production have made them the subject of work by research universities, the USDA, and many beekeepers and commodity organizations that have invested significant effort and money to study honey bee evolution, biology, genetics, foraging behavior, communication strategies, and management.
With the current suite of problems afflicting bees, this research is more crucial than ever. As a result of these concerns, we are learning more than ever about how bees live and work, and the factors that affect their populations.
ACTING AS ONE
Honey bees are social insects that live together in large numbers. This is in contrast to solitary bees (including sweat bees, leafcutter bees and others) and most other insects. In the case of solitary bees, the female is responsible for building the nest, collecting food, feeding the young, and defending the nest.
Honey bees evolved differently, and now large numbers of sterile female worker bees act as a “super organism,” that is, an individual bee is part of the larger social unit of the hive. A mated queen handles reproductive duties by laying eggs fertilized by sperm provided by male bees (drones) during the queen’s mating flight. While reproductive functions are important, it is the female workers (as sisters and daughters of the queen) who perform the majority of tasks inside and outside of the hive.
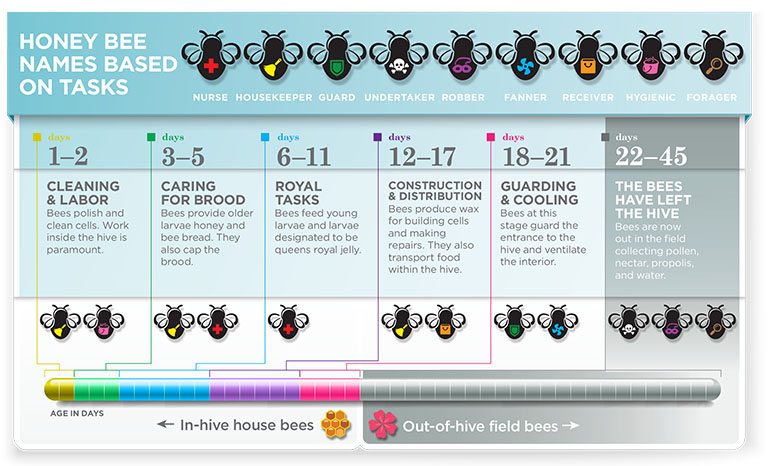
Honey bees cannot survive long on their own outside of the colony. Worker bees perform specific jobs based on their age.
Young adult workers perform in-hive tasks like feeding the larvae and queen, building wax combs, processing nectar into honey, keeping the hive cleaned, and guarding the colony.
As those adult workers age, their flight muscles develop to allow them to do out-of-hive tasks such as removing dead, dying, and diseased bees away from the colony, and, most importantly, foraging for pollen, nectar, water, and propolis. Propolis is resin from plants (including poplar trees) that honey bees use as a “bee glue” in the hive and as an antimicrobial medicine for the colony.
The oldest bees in the colony generally perform the most energetically demanding and dangerous work of foraging for pollen, scouting for forage areas, and finding new colony locations. Ultimately, these are a bee’s final jobs.
This division of labor among the adult workers is a model of efficiency, like a factory assembly line in which each worker has a defined and indispensable role. When a forager comes back with nectar, she does not deposit the nectar directly in a honey cell. Her priority is to get back to foraging. Instead, she passes the nectar to a receiver bee, who will then pass it along to others until it is placed in a cell. If the forager cannot find a receiver bee, she will buzz and recruit bees to become receivers. Once the forager bee has transferred the nectar to the receiver, she can immediately return to her duties finding more nectar and pollen.

IT SMELLS LIKE HOME
Like other insects, bees sense odors (smell) with their antennae. Every colony has its unique chemical “signature” smell that allows the guard bees to recognize that the incoming foraging bees belong to that specific colony, and are not foreign bees seeking to rob honey.
Within a hive, the bees’ bodies secrete a hydrocarbon blend onto the bee’s surface. The hydrocarbon also picks up particular floral, wax, and propolis scents that set them apart from other bees in the area — even those bees that may be from an adjacent colony just a few feet away in a large apiary that contains thousands of hive boxes.

AN INFORMATION PROCESSING CENTER
With a workforce consisting of more than 40,000, it is imperative that a hive is able to clearly and rapidly communicate the needs of the group — the same is true even for smaller colonies of a few thousand bees. Given that the inside of the hive is dark, the only true way of communicating is by olfactory (smell) cues and vibrations.
Pheromones are chemicals that bees release and can detect with their antennae. The bees then react to the specific messages these compounds convey. Pheromones can communicate the presence of the queen (queen pheromone), the queen’s health, what and when to feed developing larvae, and when an attacker is threatening the hive (alarm pheromone).
DANCING FOR DIRECTION
Like humans, honey bees have a symbolic language — bees use their famous “waggle dance” as a language. These dances allow a returning forager to communicate with its hive mates about the location (direction and distance) of pollen and nectar. Scientists have identified two different dance languages: the round dance and waggle dance, for which Karl von Frisch won a Nobel Prize in 1973.
The round dance happens when nectar and pollen resources are close to the hive (within about 100 feet). The forager bee will turn in circles alternating to the left and right. While this conveys the message that flowers are near, the round dance does not provide information about direction.
While dancing in a circle, the forager passes off small amounts of nectar to several different foragers. Not only can the newly recruited foragers taste the nectar, they also pick up the smell of the flowers from the forager’s body. Taste and smell are valuable forms of information that become a signature of what the foragers are searching for around the hive. Incidentally, this is the primary reason that flowers smell the way they do — while not all flowers are pleasing to humans, their various odors include a range of compounds that attract a diverse group of potential pollinators.
As the distance to the food source gets farther away from the hive, the round dance coalesces into the waggle dance. The waggle dance communicates information about both the distance and direction of the flowers.
Inside the darkness of the hive on a vertical comb, the returning forager who has information to convey will dance in a pattern close to a figure eight. On the vertical comb, the bees interpret the direction of the sun to be always at the top of the hive. So, if the source of the nectar is 90° to the left of the sun, the forager performing the waggle dance will orient her head in that direction with respect to straight up. Bees can tell which way is up using gravity and they can follow the dancer by feeling the wind of her buzzing wings. The foragers, responding to this information, will leave the hive at an angle of 90° to the left of the sun and travel in the general direction of the food source.
If the flowers are two miles from the colony, the bee will orient to the direction and vigorously shake and vibrate. If the food source is one mile away, she will shake and vibrate faster for a shorter duration than if it is two miles away. The length of time that the forager waggles conveys to the other workers how far away they will have to search.
The dance does not pinpoint exactly where the plant patch is, but it provides the general location of where to slow down and search. Once in the general vicinity, workers will rely on visual and olfactory cues to locate the flowers. The overall vigor and intensity of the dance is also a cue — it excites other bees when a forager is more animated — and will influence the numbers of foragers recruited.
BEES REMEMBER
Amazingly, a forager remembers where she has been. She has a mental map in her brain that allows her to return to the same place she fed the day before and that allows her to find her home when she is done.
A honey bee’s compound eyes do not see with as much resolution as human eyes — researchers believe that what a bee sees is akin to a very crude pixelated image. But that doesn’t mean visual cues are unimportant. Bees can and do see, learn, and recall a variety of landmarks and cues, including floral smells, shapes, patterns, and colors. They are also very good at perceiving movement. As a bee gets closer to the flower patch, visual clues take over. Color (specifically ultraviolet (UV) light) is another very important cue. Her eyesight is good enough that when she is flying she can discriminate familiar but general shapes and patterns.
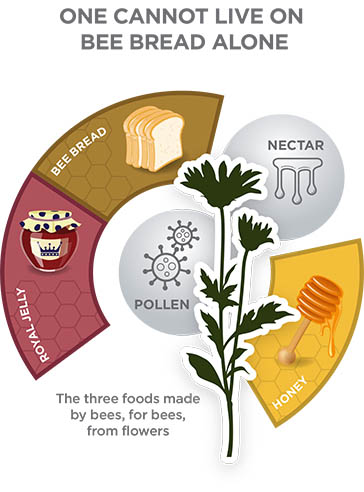
THE BEE FACTORY PRODUCES BEE BREAD AND HONEY
Most organisms need a diverse diet consisting of minerals, carbohydrates (sugars), fats, and amino acids (proteins) to survive and reproduce. Honey bees get these essential nutrients from flowers. Nectar (produced by the glands of flowers to attract pollinators) is rich in sugar and becomes the bee’s main energy source. Flower pollen is the main source of amino acids that make up proteins (and some fats) that provide the building blocks in the bee’s body.
Honey bees are willing to eat fresh nectar (which has far more water and less sugar than honey) and pollen as it comes into the hive. However, altering nectar and pollen gives them flexibility in storing these products for those inevitable times when pollen and nectar become scarce in early spring and late fall.
CARBOHYDRATES AND NECTAR
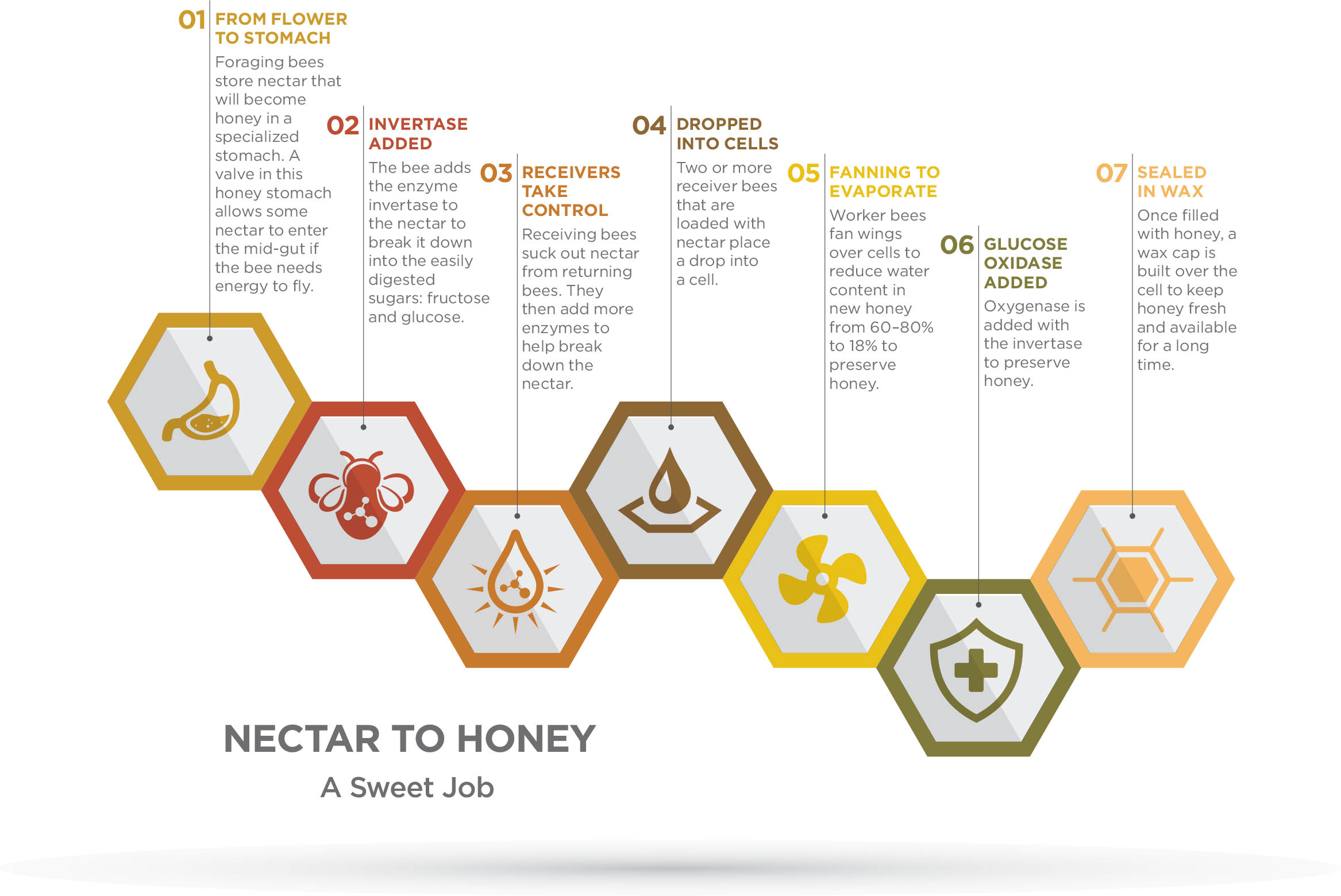
Honey is not just a repository of concentrated nectar. It is a manufactured product that uses nectar as the primary ingredient. The process of converting nectar into honey begins with the foragers.
Foraging bees lap up minute amounts of nectar from flower glands located at the base of the female portion of the flower or from extra-floral nectaries (depending on plant species). That foraging bee will digest some of that nectar for its own energy needs. But most of the nectar will remain in the bee’s honey stomach, an organ that is adapted for transporting nectar and bringing it back to the colony for eventual storage. The foraging bee visits flowers until she fills her honey stomach, and then either continues foraging until she has a full load of pollen or simply returns to the hive.
While the nectar is in the honey stomach, the enzyme called invertase begins to break the more complex sugars (such as sucrose) into glucose and fructose, which are easier for honey bees to digest (as well as for humans and other animals). Upon returning to the hive, the forager opens her mandibles and allows a receiver bee to suck out the regurgitated nectar. The forager usually transfers the nectar to two or more receivers who then place a drop of nectar into a cell. The process of exchanging food like this is called trophallaxis. During the process of transferring nectar from one receiver bee to another, the bees add other enzymes to help break down the original nectar and stabilize it against degradation. Nectar is 60 to 80 percent water, depending on the plant species. Conversely, honey is less than 18 percent water — illustrating another way bees refine nectar. Honey’s lower water content helps preserve it. Worker bees remove the water by fanning their wings in the hive to evaporate as much water as possible, effectively acting as tiny dehydrators. Bees don’t fan their wings at each and every cell, but the passing of air throughout the hive helps evaporate the water.
Reducing the water content also increases honey’s sugar content, so it provides greater energy per unit volume than the more dilute nectar. Given that honey is the “high octane” fuel for the extremely active bees, the more concentrated the product, the more energy and bang-for-the-buck that the bees obtain.
Removing the water from nectar is not the only way honey bees preserve nectar. They also add preservatives to prevent honey from spoiling. Like humans, bees must constantly battle the ubiquitous bacterial and fungal organisms that cause food to spoil. Honey bees add an enzyme called glucose oxidase (a type of oxygenase) to make honey mildly antiseptic and sterile. Glucose oxidase works at the honey’s surface where it combines with oxygen to turn glucose into gluconic acid and hydrogen peroxide.
Honey also has such a high concentration of sugar and such low water content that it draws moisture from any microorganism that lands in it. These features, combined with the fact that honey is acidic (with an average pH of 3.8), makes it highly unlikely that microorganisms can survive in this hostile environment.
Once bees fill a cell in their comb with honey, the bees build a wax cap over it. Over time, the cap turns darker as the air escapes. At this point, the cell is considered sealed, and the honey inside may remain edible and unspoiled for a very long time.

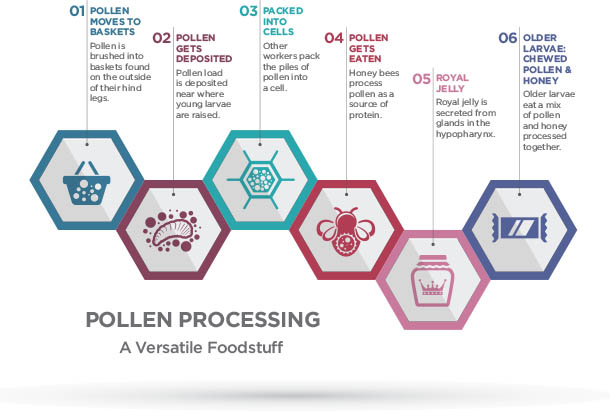
Pollen from the anther, or male part of the flower, is a colony’s protein source. As bees fly, they become electrostatically-charged. As a result, when bees land on a flower, pollen literally “jumps” off the anther and attaches to the hairs on the bee’s body. These hairs are also branched, which helps hold pollen. This adaptation makes the business of collecting pollen easier.
However, bees must still considerably manipulate the plant structure to get enough pollen for a full load. As the bees work the flowers and collect the pollen on their bodies, they will brush pollen into a pollen basket (corbicula) found on the outside of her hind legs. Once a bee fills both baskets with pollen, she returns to the hive.



Instead of passing pollen to other bees like they do with nectar, a foraging bee that returns to the hive with a loaded pollen basket will deposit her load somewhere near where young larvae are being raised. Young nurse bees are the principal consumers of stored pollen (called bee bread), which they feed to the larvae.
Bees also enzymatically process and age the bread. Other bees will pack the pollen into a cell. After the pollen forager has completed her task, she may consume some honey for energy and then return to the foraging site to retrieve more pollen.
Honey bees can eat pollen like they eat nectar, but pollen’s outer coating can be difficult to break down and digest. Young adult honey bees (nurse bees) turn pollen into a more easily digestible product called bee bread. Nurse bees digest bee bread in their guts, and then glands in their head (hypopharyngeal glands) use the digested protein to produce royal jelly or brood jelly. Older worker larvae are fed a mixture of chewed up pollen and diluted honey in addition to brood jelly; however, the queen is fed royal jelly throughout her life.

Saving for a Rainy or Cold Day
Bees store honey and pollen so the colony has food when flowers are in short supply, on rainy days when foraging activity is limited, or through the winter when the bees are clustered in the hive for months on end.
For an organism with a colonial life cycle and enormous energy needs, the ability to store food resources isessential. Without it, honey bees would periodically run out of food and starve to death.
We all know that during the spring and fall, flowers do not bloom on the same date each year. In fact, when flowers bloom can vary by weeks from year to year. This is why a hive needs thousands of foragers to take advantage of flowers when they are blooming. The next flush of flowers and weather are both unpredictable in timing and abundance, so gathering the products from flowers is critical when they are available.
The fall nectar flow is particularly variable from year to year. Honey bees depend heavily on the late-season rush of goldenrod, asters, and other plants in the fall. Many beekeepers usually harvest most of their honey in August and trust that the colony will collect additional nectar to get them through the winter. Most beekeepers will also supplement colonies with sugar water during this time. Still, this last flush of flowers is critical to provide the colony with enough stored honey to help them survive the winter.
Drinking at the Mud Hole
Honey bees gather minerals and a variety of salts from shallow and often stagnant water puddles. It sounds counter-intuitive, but the pristine and clear waters of a babbling brook are less appealing to honey bees. Bees prefer brackish and mucky waters because they contain a range of micronutrients not easily found during their visits to plants.
A Room with Six Walls
Each honey bee has glands on the lower side of its abdomen that secrete a clear liquid wax. After a few minutes, this material solidifies but remains pliable by chewing. Working the wax in their mouths, workers build the comb walls tilted slightly upward to keep the honey from flowing out of the cell.
Hexagonal cells are characteristic of a honey bee’s comb. A hexagonal cell with its six walls has been shown to be the most efficient way of using all the space available. The six-sided cell also maximizes strength, while minimizing the amount of wax needed for building the walls. When a frame is completely full of honey or pollen, it can weigh several pounds, and yet the hexagonal design doesn’t sag or stretch.
Bees must expend a lot of energy to produce wax. It takes eight ounces of honey combined with secretions from the bees themselves to make just a single ounce of wax. Beekeepers reuse and recycle all wax cells to reduce the energy they must expend to make wax, which then allows the bees to store more honey. This is one of the reasons that commercial beekeepers are loathe to throw out old frames. To maintain a clean, healthy operation, it is sometimes necessary to throw out a frame, especially if disease, parasites, and pesticide contamination are suspected.
An Air-conditioned Hive
Honey bees require very specific temperatures inside the hive to protect their brood and food reserves. If the hive temperature gets too hot (this is common in the summer), foragers will collect water and bring the water back to the hive in their honey stomachs.
Foragers bringing water back to the colony transfer it to receiver bees who put small droplets into empty cells, or close to individual brood cells, but do not touch the larvae or eggs. Workers at the entrance will fan their wings to create air currents inside the hive that transfers the heat into the water, which is an evaporative cooling process.

A Call to Arms
Honey bee colonies represent an incredibly rich and uncommon food resource. Pounds of honey and thousands of vulnerable and protein-rich larvae inside the hive make a tempting target for any animal brave enough to tear into it. Honey bees will aggressively defend the resources they have worked so hard to collect, process, and store because their future depends on them. In fact, the venom in their sting is a potent deterrent to many mammals, although it is sometimes insufficient to completely keep them at bay.
Workers known as guard bees defend the entrance against marauding insects and mammals that try to gain access to the hive’s contents. Marauders can include honey bees from other colonies (robber bees), many species of yellow jackets, as well as, mice, skunks, raccoons and bears. Honey bees must be particularly vigilant when foragers from other colonies attempt to rob honey after the last fall flowers have bloomed.
Guard bees defend the hive against insects such as wasps by biting and stinging them. Against larger invaders such as mice, honey bees rely on an accumulation of workers to sting the animal.
An adult honey bee worker has a serrated stinger, which is intended to lodge in its target. When a honey bee stings, it is a one-shot proposition (unlike wasps and many ants). The honey bee loses its stinger and the venom gland from inside its abdomen, then dies shortly afterward.
The embedded stinger continues to pump venom, appearing like a very tiny beating heart. But perhaps more importantly, it smears the area with an alarm pheromone that alerts other bees of immediate and impending danger, and it marks the spot for others to sting. That’s why one sting often leads to more in short order. If you’ve ever been stung by a bee and taken the time to observe the spot, you will see the gland on your skin. If you carefully scrape off the stinger and venom gland (as opposed to crushing or swatting at it), less venom will be pumped into the site of the sting.
Bees may have poor eyesight, but they have excellent olfactory (odor) perception, so they can track invaders even as they run away — if you have ever been chased by bees, you know this first-hand. The alarm pheromone recruits more members of the hive to come to the site of the “battle” and offer their assistance.
Airlifting the Dead, Deformed, Diseased, and Dying
It is common for dead, deformed, diseased, and dying bees to be found in or near a hive by other colony residents. A group of bees quickly identifies and drags these bees out of the colony — sometimes close to the entrance, but often farther away.

The bees that do this job are called undertaker (or mortician) bees. Sometimes, undertaker bees actually pick up their dead sisters and fly away with them. They remove them from the hive for hygienic purposes — to get them away from the colony where healthy bees will not come in contact with them.
When dead bees are deposited at the entrance, they are often consumed by ants, yellow jackets, and other scavengers.

Building and Maintaining a City of Wax
Beekeepers learned many centuries ago that bees could be housed in hollow logs, clay vessels, and woven-domed straw baskets (called skeps). The problem was that removing the wax and extracting the honey meant the container had to be destroyed, because the combs were haphazardly positioned within these containers. Inevitably, some combs with honey would go to waste.
So, for harvesting honey, the invention of the Langstroth hive, which was patented in 1852, was an important advance for beekeepers. Lorenzo Langstroth was a Philadelphia theologian who built a wooden honey bee hive with removable frames that was simple to assemble, inexpensive, and relied on materials that were portable and reusable.
Langstroth and others observed that honey bees filled in spaces that were either smaller or larger than the width of one bee. If the space was too small for the bees to pass through, they would seal the space with propolis. If the space was too large, the bees add comb to fill the larger space.
In either case, the frames become attached to one another, which prevented the beekeeper from readily pulling individual frames out of the hive. This optimum space became known as “bee space,” and when Langstroth built his hive, he made sure the frames were spaced apart by the width of one bee (3/8 inch), which allowed the frames to remain largely unattached to each other.

The Langstroth hive’s many benefits include:
• Removable frames that allow beekeepers to easily harvest honey
• Reusable frames that allow honey bees to reuse the wax in their comb
• A scalable system that makes it easy for beekeepers to add boxes on top to provide bees more space to store honey
• A way for beekeepers to inspect bees without disrupting the colony
• A simple screen (called a queen excluder) that can be used between boxes to keep honey and brood frames separate
• The ability to fill frames with a healthy brood as “transplants” to start a new hive or reinforce a weaker hive
• A way to readily transport colonies from one location to another, which allows beekeepers to move bees from coast to coast for commercial pollination services
The Langstroth hive is largely unchanged from its original design. The adoption of the Langstroth hive is credited with creating the commercial bee industry and making it popular for hobbyists to raise honey bees.
Drawing Your Home’s Blueprint on a Foundation
Each wooden frame encloses a plastic or wax foundation. It is this vertical foundation that lets beekeepers control where the bees build their honey combs. Without the foundation, bees will build combs in whatever direction that makes use of the available space, which means beekeepers could not remove each frame without damaging the combs. An additional advantage of using a plastic or wax foundation is that it gives the comb structural stability and support when filled with honey.
DRAWING YOUR HOME’S BLUEPRINT ON A FOUNDATION
The bottom board provides a landing pad and an entrance-exit from the hive. It also provides a platform for bees to ventilate the hive during the hottest days of the year.
You can think of the brood box as the hive’s nursery. It consists of one or more boxes where the queen lays eggs and workers feed developing larvae. Beekeepers do not harvest any honey from the frames in the brood boxes.

QUEEN EXCLUDER
The metal or plastic queen excluder prevents the queen from leaving the brood box to lay eggs in the upper supers. The frame is wide enough for workers to pass through, but not the queen.

SUPER
Honey supers are boxes similar to brood boxes, but are typically shorter.
Each super holds nine to 10 frames to store excess honey. As bees fill each super, beekeepers add more supers. Beekeepers harvest honey from the supers — each weighs 35 to 40 pounds when filled with honey.

INNER COVER
The inner cover is the hive’s ceiling. An elliptical hole in the center provides ventilation. The inner cover provides the proper bee space so bees do not glue it to the outer cover with propolis.

OUTER COVER
The outer cover is the roof that protects the colony from rain and snow.

SMOKING
Beekeepers use smoke to disarm the guards who recruit bees to sting. The light smoke masks the alarm pheromone, so fewer bees are recruited to sting. It also induces the bees to engorge themselves with honey, which makes them more docile and less likely to sting.

A healthy colony averages between 20,000 to 60,000 honey bees, but these estimates vary depending on the availability of nectar and pollen, time of year, and health of the colony. Within the colony, honey bees are divided into three biologically and physiologically distinct adult castes:
1. A single reproductive female known as the queen
2. Several hundred to a thousand reproductive males called drones
3. Thousands of sterile female workers
While there are differences between the three castes, each passes through the same phases of development: egg, five larval stages (each stage is called an instar), a pupal stage, and adult. The number of days to go from an egg to an adult varies by caste.
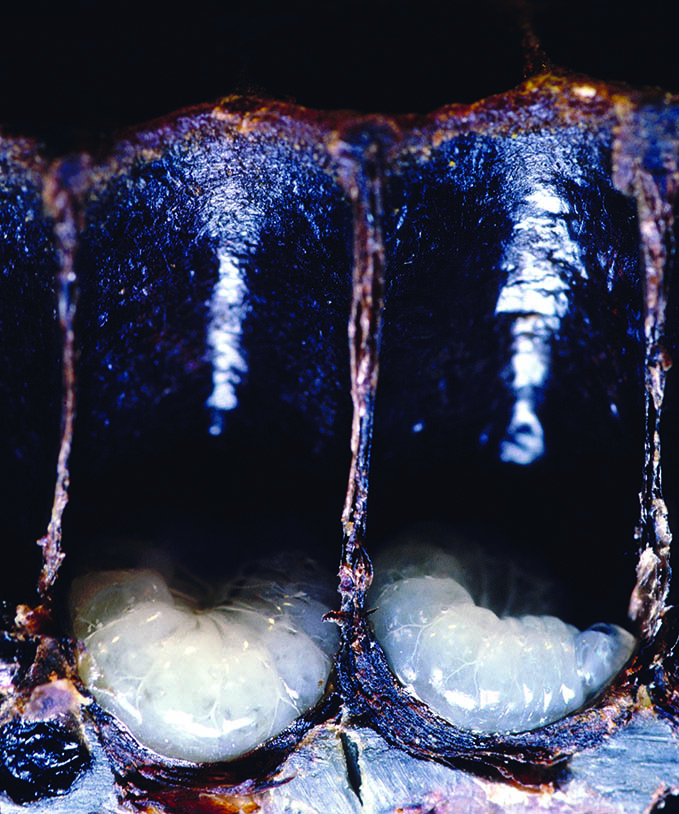
Over the course of six days, each larva increases in size 1,500-fold from the first to the fifth instar stages of development. Part of this rapid growth is due to the larvae receiving exceptionally rich food during that time. Unlike caterpillars and other immature insects, honey bee larvae do not have to find (or even chew) their own food — their food is optimized so that there is very little processing required for them to extract nutrition from it.
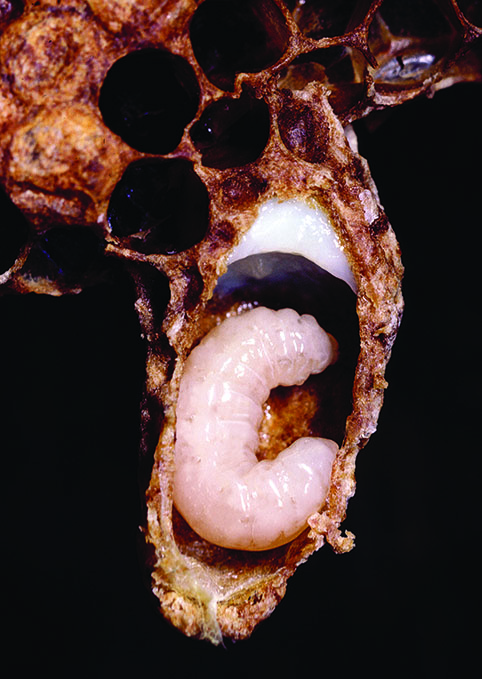
Just before the fifth instar, a larva is ready to transform into a pupa. The larva (called a prepupa at this point) weaves a fine cocoon, and worker bees cap the cell with a layer of wax. Pupae do not feed while they are undergoing the transformation (metamorphosis) from a larva devoid of appendages to an adult worker bee with six legs and two pairs of wings. As the adult bee emerges (ecloses), she will clean her cell so it can be reused.
Food Quality Determines Who’s Who
Regardless of caste and sex, all larvae are fed royal jelly for the first three days after emerging from the egg. Royal jelly is a milky fluid rich in protein and lipids that is produced by the hypopharyngeal gland located in the head of young nurse bees. Nurse bees regurgitate royal jelly into the cell with the developing larva. The larva floats in its food as it imbibes the royal jelly. The larva does not drown, because it has breathing holes (spiracles) along both sides of its body and one side is always up.
After those first three days, the type of food the larva receives determines whether it will become a sterile female worker or reproductive queen. Larvae fully fed an unlimited supply of royal jelly will become queens and have fully functional ovaries. Those destined to develop into sterile females with underdeveloped ovaries and drones are kept on a diet of brood jelly and bee bread (a mixture of pollen and diluted honey). A suite of pheromone cues within the hive determines the amount of royal jelly that workers feed developing larvae (that is, whether they will rear new queens or workers).

A Single Queen
Regardless of the colony’s size, there is generally only one egg-producing queen in the hive at any one time. The colony periodically rears new queens as a means to disperse and develop new colonies and to replace aging and failing queens. It is primarily the type of brood cell that tells workers whether to feed the larva food that is appropriate for a worker or royalty. Queen cells start from wax cups that point down and the bees elongate the cell as the queen grows to a shape that resembles a peanut.
A productive queen lives two to four years. She is distinguished from the workers by her large size. In particular, a queen has an enlarged abdomen that extends well beyond the wings and a wider thorax.
The queen starts laying eggs in late winter or early spring. Nurse bees start eating pollen that was stored in the fall, which is an essential ingredient for making protein and lipid-rich royal jelly. Feeding the queen royal jelly stimulates her to lay eggs.
When fresh pollen is available in the spring from plants (such as maple trees) the brood rearing rate increases greatly. This begins a spring colony buildup during which the queen can lay 1,500 eggs per day.

Drones
There are usually 600 to 1,000 drone bees in larger colonies. Colonies produce an abundance of drones each spring. These male reproductive bees are rather unique in that they hatch from an unfertilized egg laid by the queen.
Drones have no father and their genetic makeup comes only from the queen. When the queen mates with many drones during a mating flight, she stores their sperm in her spermatheca, a round organ in her abdomen. The queen can control whether an egg that is about to be laid is fertilized. She measures the cell prior to laying an egg inside it. If it is a larger, drone-sized cell, she does not open the valve to allow sperm to enter the egg. The egg will then hatch into a drone larva.
Drones are not individuals in the colony workforce. Their sole purpose is to congregate outside the hive, in drone congregation areas and mate with newly-emerged virgin queens from nearby colonies. Drones use their keen eyesight to find females while in flight. A drone is easily identified by the large eyes that cover much of its head and meet at the top (unlike females that have a space between their eyes).
Drones cannot sting, forage, or help in any colony tasks. They are a drain on colony resources and are useful only during mating, which occurs while the weather is fair. So, when temperatures drop in the fall, workers force the drones out of the hive where they will die of starvation and cold.

Workers
A colony’s success depends on sterile female workers that perform virtually all of the work. While foragers are seen gathering nectar, pollen, water, and propolis, they only represent about 20 to 35 percent of a colony’s total workers. The remainder of the colony performs key tasks within the hive.
A worker bee’s lifespan varies with the time of year. During most of the year, when the brood nest is expanding and foragers are bringing nectar and pollen back to the hive, a worker bee may live six weeks. During the winter (when activity is much slower) a worker bee can live for five or six months.
When rearing the brood, workers maintain a hive temperature of about 93°F. Young workers clean cells, feed the queen, remove debris, and add wax to cells. Whether it’s feeding larvae, keeping the hive clean, or doing the multitude of small tasks involved in colony maintenance, these younger adult bees reliably transition from one job to the next.
But there is also flexibility (referred to as plasticity) in these task schedules.
For example, if an off-target pesticide application kills a large number of foragers, young bees may become precocious foragers. If all of the nurse bees are removed from a colony, the hypopharyngeal glands of older bees will become active and the bees will revert to nursing duty.
At about three weeks old, a worker bee that has spent the entirety of her life within the colony is ready to take on her final and most dangerous task: that of a forager working outside of the hive. New foragers begin by performing orientation flights in late afternoon around the hive.

Hundreds of these soon-to-be foragers fly close to the hive, memorizing everything around them in a mental map of where the hive is located. Within a few days following the onset of these orientation flights, the foragers start scouting for pollen, water, nectar, and propolis.
For the next couple of weeks, the forager will travel back and forth to the hive covering many miles. Individual foragers will specialize in collecting either pollen, nectar, propolis, or water. However, foragers can switch duties — collecting pollen one day, then water the next, depending on the information she receives from the colony.
If a returning forager is inundated with receiver bees, she not only gets unloaded quickly, she also receives positive reinforcement that induces her to continue foraging for whatever it was she brought in to the hive. Additionally, she might perform a bee dance to recruit more foragers. This is how the colony communicates whether their provisions are in short supply, or relatively abundant, and foragers change their foraging patterns accordingly.
The majority of honey bees have a typical round-trip flying distance of two to four miles, and travel at a mean velocity of about 15 miles per hour. Bees can use the nectar in their honey stomachs much like a gas tank.
If a nectar forager needs energy as she is travelling, she will refuel in flight by opening a valve in the honey stomach that releases nectar into the midgut.
Foraging is physically demanding and carries a tremendous physiological cost for the honey bee. After a few weeks of foraging, a honey bee forager has worked herself to death.

ONE COLONY BECOMES TWO
A healthy colony works as a team — some individuals take care of the colony while others forage for provisions. Depending on nectar flow and colony population size, a colony can fill a honey box or “super” in as few as three days. When the hive becomes full of brood, honey, and bee bread, there is simply no space left to expand. When space is limiting, it can induce honey bees to swarm, effectively dividing the group in two.
The existing queen has mandibular pheromone (sometimes called the queen substance) that inhibits the construction of queen cells. As long as the queen is healthy and laying eggs, this pheromone prevents the hive from developing new queens. This pheromone also suppresses ovarian development among the sterile worker females.
Workers do have a couple of ovarioles, whereas the queen has hundreds. Most of the workers’ ovaries are underdeveloped, so they do not produce eggs. If a worker with developed ovaries lays an egg in a cell, her sisters will recognize the egg is not from the queen and they will eat it. However, if the queen dies and they are unable to raise a new queen, some of the workers’ ovaries will develop and the workers will lay unfertilized eggs (drones). Eventually, this will lead to the death of the colony, since the colony is no longer able to effectively generate worker bees.
A colony can also die if a queen uses up her supply of viable sperm. If that happens, the queen will start laying unfertilized eggs (referred to as a drone queen), which produce only drones that do not forage or care for the colony.
In Indiana, swarming happens mostly in May and June. As a queen ages, she produces less mandibular pheromone. Under crowded conditions, the mandibular pheromone is not well distributed through the hive. With less pheromone to inhibit queen rearing, workers are signaled to start raising queens, and the colony division process (swarming) begins about two weeks before the old queen leaves. The workers put the old queen on “a diet” to slim her down and allow her flight muscles to develop in order to fly with the swarm.
Before swarming occurs, workers usually start five to 25 queen cells. Any fertilized egg or larva can develop into a queen just by providing it a different quality of food (royal jelly).
Workers closely watch the timing of when the old queen leaves and the new queens emerge. Sometimes, the workers hold the replacement queens as captives inside their cells until the old queen has left with the swarm. The workers create vibrations on the cells to inhibit virgin queens from emerging prematurely.
The colony requires an orderly process to initiate swarming, the old queen must leave and a substitute virgin queen must be ready to replace her as soon as possible thereafter. Two queens will not co-exist in one colony; only one will inherit the colony. But sometimes, a large colony will send another swarm, an “afterswarm,” with a virgin queen, where a small number of bees remain in the nest with a second virgin queen.
Just before swarming, honey bees leaving with the queen gorge themselves with honey. When a colony swarms, the old queen takes about half of the colony with her. The swarm will cluster on a branch and send out scouts to look for suitable cavities. Swarming is a risky business, and most swarms do not survive their first winter simply because they cannot build up sufficient colony numbers and resources to last through the winter.
MATING ON THE WING
At most, a queen only leaves the hive twice. One is when she swarms as the established queen; however, this may not happen during her lifetime. All queens depart from the hive to mate. After about seven days living in the hive, the new unmated queen makes an orientation flight to familiarize herself with the hive’s location. Soon after, the virgin queen leaves the hive on an afternoon to find the drones.
Drones usually take mating flights in the afternoon. Drones fly to specific congregation areas where multiple males wait for virgin queens to appear. The drones will fly for about 45 minutes before returning to the hive to refuel with honey. Drones may repeat this process multiple times during the day. The vast majority of drones never mate.
On her mating flight, a queen flies toward a drone congregation area. Once there, the queen’s pheromone attracts the attention of the males. She will mate about 60 to 100 feet off the ground with 12 to 18 drones. Each male will grasp onto the queen and evert their aedeagus (penis) to mate with the queen in flight. The drone will die soon after mating is complete. The queen returns to the colony, which she will not leave again unless it is time for her to swarm.

After the mating flight, the sperm from the multiple matings is mixed together over 48 hours inside the queen’s spermatheca, a round organ that stores sperm near the tip of the abdomen. The new queen’s spermatheca contains about 8 million sperm that she can use to fertilize all of the eggs that she will lay over the remainder of her life.
Beekeepers have developed methods to artificially raise queens to replace lost queens, so that managed colonies remain “queen right” because virgin queens can be killed on mating flights. In addition, artificially inseminating queens provides beekeepers with the ability to control colony genetics.
The queen lays her first eggs in the brood chamber close to the center of the comb. She will deposit a single egg in each cell. Each egg looks like a tiny grain of rice and has an opening on the side to allow sperm to penetrate. Eggs that are fertilized with sperm become either queens or sterile female workers, while unfertilized eggs become drones.
A queen that runs out of sperm is either very old or did not mate with sufficient males. If this occurs, she will lay only drones and must be replaced, either by her nest mates or by a human caretaker. If an infertile queen persists, she will continue to produce drones and the colony will die, because she can produce no new workers to sustain the colony.

THE WINTER CLUSTER
Winter can be the final stressor that finishes off unhealthy bees and weak hives. It is typical for 15 to 28 percent of all hives to not survive the winter. There are two factors that allow bees to endure frigid winter temperatures:
1. Enough stored honey that the bees can eat
2. An adequate number of bees to cluster and keep the overwintering colony warm
If the colony is insufficiently strong going into winter, or it does not have enough provisions to last through the winter, its chances of survival are greatly reduced. Second to Varroa mites, winter starvation is believed to be the most common cause for colony mortality.

Honey bees do not hibernate. Because they are small, a bee’s internal temperature will rapidly take on the outside temperature, which can be lethal during a North American winter. However, Western or European honey bees have adapted a survival strategy of clustering together in a mass to generate and conserve heat.
In late fall, a bee colony contains about half the number of adult workers it had in the summer, but there is no brood to care for, and foraging is complete for the year. The colony’s goal heading into winter is to maintain sufficient temperatures to keep the colony alive. Bees accomplish this by forming a tight ball (cluster), which is interrupted by wax combs that provide insulation.
On the outside of the cluster are a mantle of bees that connect their legs together and essentially form a shell. These bees serve as a heat shield to help retain the heat within the cluster. In the interior, workers perform “isometric exercises,” while remaining virtually motionless and vibrate their thoracic muscles. Shivering their muscles generates heat.
Individual bees take turns heating themselves to almost 100°F. Other bees will fan their wings to disperse excess heat, and regulate the amount of carbon dioxide in the cluster. The individual bees take turns heating themselves up, fanning, and serving as mantle bees, rotating in and out of the ball with the queen safely in its center.
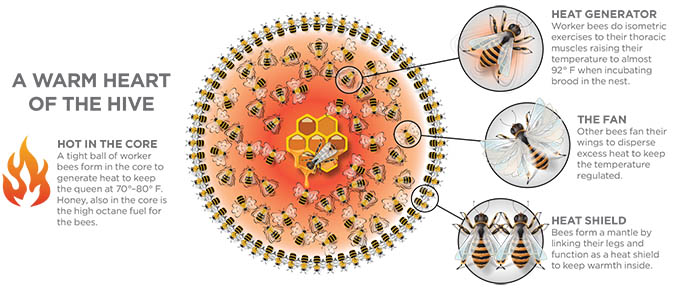
They do not heat the whole hive, but focus on keeping the core of the cluster (where the queen is) at 70-80°F. When the bees begin rearing brood again in late winter, they will need to increase the temperature of the hive to 93°F. Generating all that heat requires more honey to fuel the energy the bees are expending. So, stored honey is located nearby the cluster. The cluster will stay in one area until they have depleted the honey reserves. Without food, the bees are forced to move to a new area of the comb that contains honey.
Honey bees that experience extended periods of cold can still face serious problems. If -10°F temperatures persist for weeks on end, bees can become chilled to the point that they are unable to move to the comb to where honey is stored. Prolonged cold can kill the cluster, and bees starve to death when they are unable to access honey.
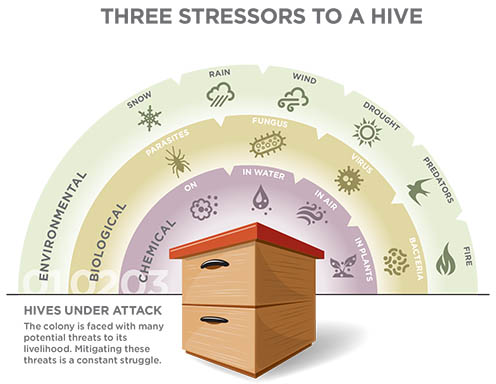
THE FOUR P’S THAT AFFECT BEES
When it comes to the factors that affect honey bees, we can look at the four P’s:
1. Pathogens
2. Pests
3. Poor nutrition
4. Pesticides
Although multiple factors (either singularly or in combination) can threaten a colony’s ability to survive, it is the fourth P (pesticides) that can be particularly challenging for bee colonies when bees forage in treated agricultural or nonagricultural sites. The colony-level effects of pesticides can vary greatly based on when the bees were exposed.
For example, in the spring and early summer, a colony is growing because nectar and pollen are abundant. So, at these times, losses from a pesticide application may not be as significant or as noticeable if the same number of deaths occur in late summer and early fall when the hive is in natural decline and the colony requires a minimum level of bees (cluster size) to keep the colony warm during the coming winter.
A colony will employ safeguards to stay viable, even after many bees have been killed. If an event kills too many foragers, the colony will have nurse bees accelerate their behavioral development and become precocious foragers. On the other hand, if pesticide residues were brought to the hive in the pollen or nectar and kills nurse bees, then some of the foragers can revert back to working inside the hive. These are biological responses that help the colony buffer itself against higher than normal losses (that is, compensate for or recover from such losses).
However, there can be serious short-term repercussions of turning nurse bees into foragers, such as having fewer workers available to rear brood, control temperatures, and clean the hive. Precocious foragers may be more susceptible to predation, because they have not had sufficient time to learn how to forage effectively, or their body physiology is not yet developed to meet the rigors that come with constant foraging. Precocious workers often have shorter lifespans and, at least initially, tend to be less efficient foragers.
As we will explore in the sections that follow, pesticide exposure is not always easy to characterize. Pinpointing a cause can be a real challenge.
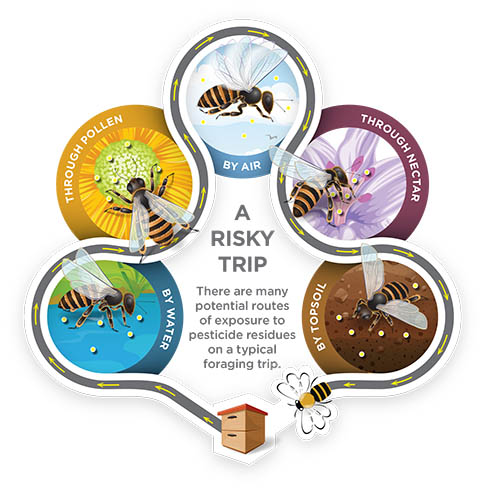
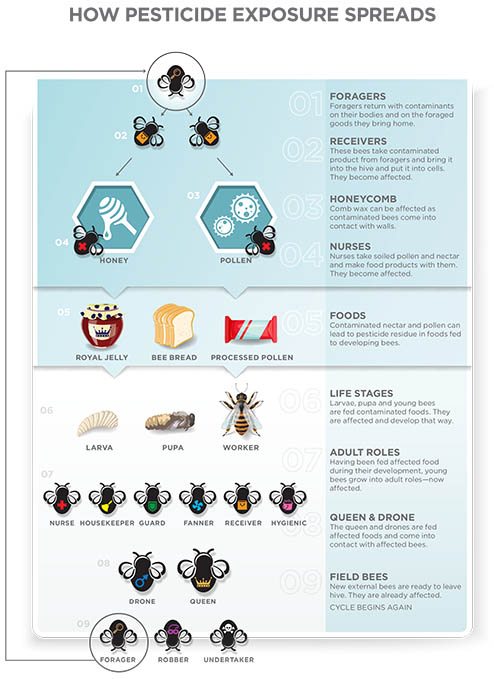
ROUTES OF PESTICIDE EXPOSURE FROM FIELD TO HIVE
The public, scientific community, and regulatory agencies have increasingly focused on the effects that pesticides have on honey bees and (to a much larger extent) the pollinator community as a whole. There are legitimate concerns for honey bees, because some insecticides will kill bees at very low concentrations.
Just like targeted insects, nontarget insects such as honey bees can encounter pesticide residues directly by coming into contact with spray droplets or dust particles during application. Bees can be exposed while landing and foraging on treated surfaces such as leaves and flowers (contact exposure). They can also be exposed to pesticides by ingesting contaminated pollen and nectar from treated plants or by ingesting water that has been contaminated with a pesticide (oral exposure). Also, plants can take up certain systemic pesticides through their roots or leaves. The plant then distributes these systemic pesticides to other parts of the plant, such as pollen and nectar, which the bee can then ingest.
Whether exposed to pesticides by contact or ingestion, forager bees can move pesticide residues into the colony. The foragers serve as an unwitting route of exposure for in-hive bees (including nurse bees, the brood, and the queen) when foragers drop off a load of tainted pollen or nectar.

It is important to remember that pesticides are not just used on agricultural crops or in residential settings. The beekeeping community also uses pesticides to control pests such as Varroa mites, small hive beetles, and wax moths. Beekeepers apply some of these compounds directly to the interior of the colony to control pests, such as the Varroa mite.
The importance of each exposure route depends on several factors:
• How the pesticide was applied
• The pesticide’s properties — How long can it persist on flowers and in water? Is it mobile in plants? Is it volatile?
• How close the hive is to the treated area
• How susceptible the bees are to the pesticide (toxicity)
Within a two- to three-mile radius around the hive, and depending on the toxicity of the pesticide to the bees, direct contact with the chemical during application or contact with recently treated foliage can directly kill foragers visiting blooming crops or ornamentals in a garden. While large bee kills are uncommon, a bee kill from pesticides can devastate a colony.
Investigators often link bee deaths to insecticides applied directly to blooming landscape trees, fruit trees, and crops. Depending on the extent of exposure and the pesticide’s toxicity, bee kills can result in the outright loss of a colony or cause such significant losses to a specific caste (such as forager bees or the brood) that it considerably weakens the colony.
When pesticides are applied during bloom, residues can remain on flowers, pollen, and nectar, and be transferred to the bee through contact exposure. The amount of potential residues depends on the environmental conditions and pesticide’s characteristics.
Bees in flight can also be exposed to pesticide drift of dusts or liquid droplets in the air — the pesticide is often carried off-target by the wind. Experience with residue studies of different pesticides in pollen and nectar of multiple crops and ornamental plants show that this will depend on pesticide properties, application timing and crop/plant features. If an applicator applies pesticides during the evening when bees are inactive, some pesticides can dry before bee activity resumes the next day. The flowers of certain crops and plants are neither attractive to honey bees or they remain closed during application, which leads to low residues in pollen and nectar.
Foraging bees can bring pesticide residues into the hive and transfer them to receiver bees and other foragers through a process known as trophallaxis. When the returning forager bee regurgitates the contents of its honey stomach to its nest mates, the receiver bees place the contaminated nectar in cells to make honey. Later, adult bees will consume the honey that contains pesticide residues.
A similar exposure can occur with pollen. Nurse bees consume more stored pollen (bee bread) than any of the adult castes in the hive. They use bee bread to produce royal jelly and brood jelly, which in turn is fed to the developing larvae and the queen. If the bee bread contains pesticide residue, then the pesticide may be expressed in the secreted jelly. Because of the way bees share and store food throughout the colony, the entire colony could be exposed to pesticide residues from a single exposure event.
EVERYTHING IS POISONOUS AT SOME DOSE
The 16th century philosopher and pioneer of toxicology, Paracelsus, is credited with coining the phrase “The dose makes the poison.” This phrase remains one of the guiding tenets of toxicology.
But it’s worth quoting Paracelsus more accurately here: “[A]ll things are poison and nothing is without poison; only the dose makes a thing not a poison.”
This full statement recognizes that all organic, inorganic, natural, or synthetic chemicals (even water) are capable of being toxic at some dose. A toxic dose differs from one chemical to the next because of how the chemical is applied, its mode of action, and whether the chemical can be metabolized and/or excreted.
When it comes to pesticides, there is always an exposure level that will not measurably affect bees. But as pesticide exposure increases, bees will begin showing sublethal effects — not unlike a person affected by a drug or disease. These effects are often expressed as impaired behavior — their activity level may greatly increase or decrease, or they may feed notably less. Increasing the exposure level can lead to death, which is what beekeepers and other observers most often notice.
A comparison to medicines can explain the concept that the dose makes the poison. If you take the recommended (therapeutic) dose of a medicine like aspirin, it should relieve your pain or fever. But exceeding the therapeutic dose can lead to adverse reactions and even death. A dose that is too low will have no curative effect, but a dose that is too high may kill.
Scientists studying bee biology have shown that there are many ways individual bees and the colony can be exposed to pesticide residues. As long as pesticides are used outdoors and in areas where bees are foraging, bees will come in contact with pesticide residues.
So, a more relevant question is: At what concentration (dose) will a pesticide exposure in the environment harm an individual bee or the colony?
One of the difficulties in determining the risk to bees from pesticide exposure is that bees forage widely in the landscape, and pesticide exposure can come from many sources. In addition, honey bees are social animals and the survival, growth, and reproductive success of the species is measured at the colony level, rather than at the individual bee level.
In other words, it can be difficult to determine the precise dose that a foraging bee or its colony will be exposed to.
Determining colony-level effects is complicated by a number of factors including:
• When the pesticide was encountered and how often it was encountered — was this a one-time acute exposure or a long-term chronic exposure?
• Whether the pesticide exposure was limited to contacting foragers in the field
• Whether the exposure was dietary — that is, did bees consume the pesticide in the pollen, nectar, or water brought back and stored in the hive?
These are the challenges that risk assessors face when they must determine the potential risk of pesticides to honey bees and other pollinators. In general, researchers determine how sensitive bees are to a pesticide through laboratory tests and in some cases, in field studies. The challenge is to determine whether a specific pesticide’s active ingredient:
• Adversely affects the various castes and age classes of honey bees
• Risk to individual bees poses a challenge for the inner workings of the colony
• Presents an unreasonable risk compared to its potential benefits
So, when answering the question, “Will applying this pesticide present a risk to honey bees?” we first need to define risk.
Risk is defined as the magnitude and likelihood of an adverse effect. It is important to remember that risk is a function of exposure and toxicity. You may know a particular compound’s toxicity, but if you don’t know the environmental exposure estimates, or you have exposure estimates but don’t know the toxicity, you only have part of the equation and can’t adequately determine risk.
Scientists study the biological response of honey bees by exposing the bees to varying doses of a pesticide (under laboratory or field conditions).
If the pesticide level in the environment is less than what is known from laboratory acute and chronic studies to cause no harm, then the presumption is that the pesticide will pose minimum risks to honey bees for the life stage tested (such as adult bees or larvae).
If the pesticide level in the environment is higher than those shown to produce acute or chronic effects in adult and/or larval bees, then the presumption is the product poses risk.
As we can guess from our understanding of bee biology, evaluating risks to honey bees presents a challenge. The hive is a colony of individuals that essentially acts as one individual. That means risk assessors must assess the risk that a pesticide poses to individual bees at different levels of organization (individual bee larvae, adult workers, queens, drones) plus the colony as a whole.
THE EPA RISK ASSESSMENT PROCESS
The U.S. Environmental Protection Agency (EPA) is tasked with (among other things) licensing the sale, distribution, and use of pesticides.
Pesticide is an inclusive term that describes the thousands of EPA-registered products used to manage insects, weeds, plant diseases, and others pests. Beyond that simple definition, this term has regulatory implications: The EPA must evaluate whether each active ingredient passes current health, safety, and environmental guidelines.
The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) — one of many laws authorizing the EPA to regulate pesticides — defines a “pesticide” as “any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pests; any substance or mixture of substances intended for use as a plant regulator, defoliant, or desiccant; and, any nitrogen stabilizer.”
For many, “pesticide” is a word with negative connotations, yet pesticides are no more inherently dangerous than medicines. If used properly, medicines can manage human ailments. The risk posed by medicines varies considerably, and depends upon the specific drug, its chemical composition, the dose taken, and the age of the person taking the medication. The same principles hold true for pesticides.
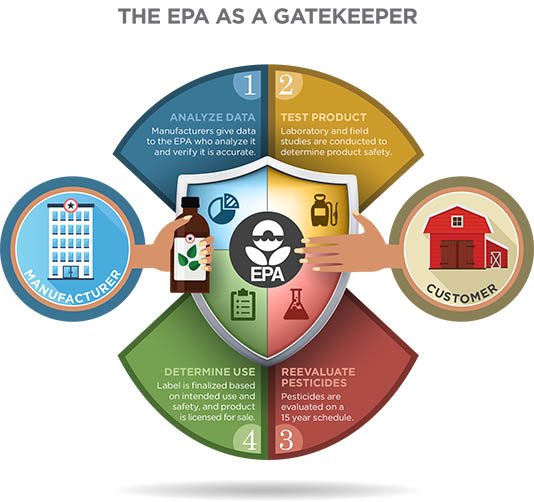
The EPA Office of Pesticide Programs (OPP) is authorized to determine whether a pesticide can be used in a manner that will not pose an unreasonable risk to human health or the environment. When a company submits a new pesticide to the EPA for review, the company (called the applicant at this stage) includes a proposed label. Once a chemical is registered, the company that owns the label is referred to as the registrant.
The EPA uses the company’s proposed label as a foundation for a formal risk assessment. Thus, it is the label that the EPA approves and registers as part of the licensing process based on a broad range of environmental fate, human health effects, and ecological effects data.
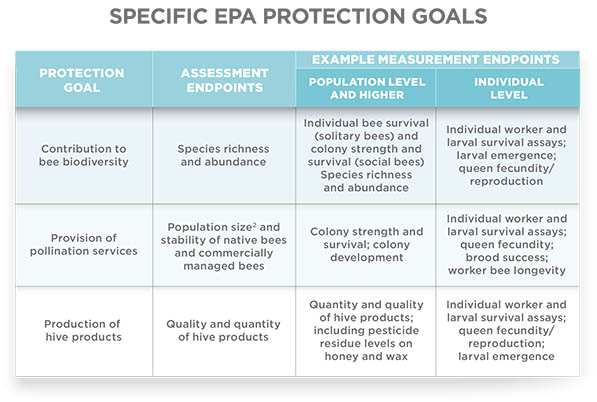
Honey bees and pollinators play an important role in the environment. That’s why the EPA convened a scientific advisory panel in 2012 to examine the procedures for assessing pesticide risk to bees. The panel was composed of recognized experts in the fields of toxicology, Apis and non-Apis bee biology, statistics, and risk assessment. The EPA also consulted various agencies, including Health Canada’s Pest Management Regulatory Agency and the California Department of Pesticide Regulation. The panel agreed that additional testing was warranted to determine the potential effects pesticides could have on non-Apis bees. They also agreed with specific protection goals (see Specific EPA Protection Goals).
The panel reviewed the procedures for how the EPA assesses pesticide risk to honey bees and other pollinators. They also suggested how to revise and add procedures to better protect these important species. In short, the EPA is always investigating ways it can refine and improve the process of assessing pesticide risk to bees.

THE HONEY BEE AS A SURROGATE FOR INSECT POLLINATORS
Even so, assessing a pesticide’s risk to insect pollinators can be complicated. It’s important to understand that the EPA doesn’t require a registrant to test the effects of their pesticide on every possible insect species. It is logistically impossible to test all species that may be exposed to a given pesticide in the environment. Some species remain unknown to science and some are endangered. Researchers do not fully know the vast majority of nonmammal species, so it isn’t possible to routinely test all these species under laboratory conditions.
That’s why it is common to extrapolate toxicity studies conducted on one organism (called a surrogate or substitute) to a broader number of related organisms. Surrogate species testing is a foundation of toxicology.
Using a single species to extrapolate the effects of pesticides on others continues to be controversial. In the case of pollinators, one frequently asked question is: How can a single species like the honey bee be a reasonable surrogate for the thousands of solitary and social non-Apis bees?
All of these species differ in their biological and life cycle traits, including body size and sociality. Honey bees and non-Apis bees may also differ in other traits such as flight season, number of generations, floral specialization, or nesting behavior. All these traits may provide different exposure routes or exposure levels among the species.
One answer to the question is that when assessing a pesticide, a researcher can extrapolate test results from individual female worker social bees to female solitary bees. Although the solitary bees do not form colonies, they do perform a variety of functions such as laying eggs, foraging, and caring for offspring. The results of testing the effects of a pesticide on the colony is thought to be equivalent to measuring the impact on social bees; however, some social bees (such as Eastern bumble bee, Bombus impatiens), may overwinter as a single organism as opposed to a colony.
A second frequently asked question is: Don’t all species of animals and plants have a different potential response to a pesticide?
On a biochemical level, species are more similar physiologically than they are dissimilar. Most species share similar physiological processes, signaling molecules, and chemical pathways that provide the rationale for why the toxic effects in a surrogate species are likely to be similar to other animal and plant species.
For instance, a pesticide that has a mode of action that affects a honey bee’s nervous system would be expected to have a similar mode of action and overt effects on other insects, including pollinators. Researchers can extrapolate effects to other species by understanding how much the indicator species shares physiological processes with other species in its group of species (called a taxon), and to some extent across taxa.
While surrogate species work well in estimating risks, there is always the possibility that differences exist between species. This is why the EPA uses other lines of evidence — such as the scientific literature and incident reports (bee kills) — to determine whether there are differences. Plus, in order to address concerns about the honey bees serving as the sole surrogate for a host of beneficial insects, regulatory authorities collaborate with researchers to expand toxicity tests to include non-Apis species. However, the same uncertainty about inter-species variability will apply to these other species if (or when) researchers can develop suitable testing protocols.
Let’s examine how the EPA currently assesses the risk pesticides can pose to bees and other pollinators.
A HOST OF QUESTIONS TO ANSWER
As part of the federal review and product registration process, the EPA bases its decisions to accept or deny a product registration based on:
• Legal mandates contained in FIFRA
• Scientific data from active ingredient-specific and product-specific tests
• Public policies that determine the acceptable risks to human health and the environment
As we’ve mentioned, honey bees are used as a surrogate to assess risks to other pollinators. Therefore, the EPA’s product registration decisions are intended to protect other insects, including social non-Apis bees like bumble bees (Bombus spp.), solitary bees like blue orchard bee (Osmia lignaria), and other pollinators like alfalfa leaf cutter bees (Megachile rotundata). As with other taxa, the EPA uses the best available information to assess the potential risks to other pollinator insects (such as butterflies).
When evaluating the potential direct risks to bees on a treated crop, regulators ask these questions:
• What crops will the pesticide be applied to?
• Are any of the target crops attractive to foraging bees?
• Is the pesticide applied during bloom?
• What are anticipated exposure routes?
For example, will bees be exposed by contact?
By ingesting residues in nectar and pollen?
• Is the pesticide water-soluble, volatile, or subject to atmospheric transport?
• Is the pesticide mobile or immobile in water and soil?
• At what rate does the pesticide break down in the environment?
• If the pesticide breaks down, are its by-products similar enough to the parent compound to cause concern? In other words, are the by-products toxic?
• At what concentrations do the laboratory studies indicate adverse effects following a single (acute) versus repeat (chronic) exposure?
• Are there data to assess the relative sensitivity of other non-Apis bees?
All of these questions revolve around knowing more about the pesticide, its proposed/existing uses, environmental exposures, toxicity, and derived benefits. Because these are serious questions that require the EPA’s diligence, the pesticide review process (for new products or modifications to existing products) is lengthy, comprehensive, time-consuming, and costly both to the EPA and registrants.
Registrants require nearly 11 years of research and $290 million to get a pesticide product with a new active ingredient developed, screened, reviewed, and registered. With the worldwide market valued at more than $50 billion, companies are willing to invest large sums of money to research their products.
The EPA uses the information it receives from each registrant to understand how they intend the product to be used. This information includes application sites (such as crops), maximum single application rates (such as pounds of active ingredient per acre), maximum number of applications, reapplication intervals, maximum seasonal/yearly application rates, application methods, and intended target pests. As noted earlier, this information is a foundation for the risk assessment.
Based on a chemical’s intended use and whether it is a new or currently registered pesticide, the EPA will develop a risk hypothesis based on the most reliable science available. In some cases, these risk hypotheses are relatively generic and presume risks across a broad range of taxa. The EPA uses data submitted by the registrant to test the risk hypothesis.
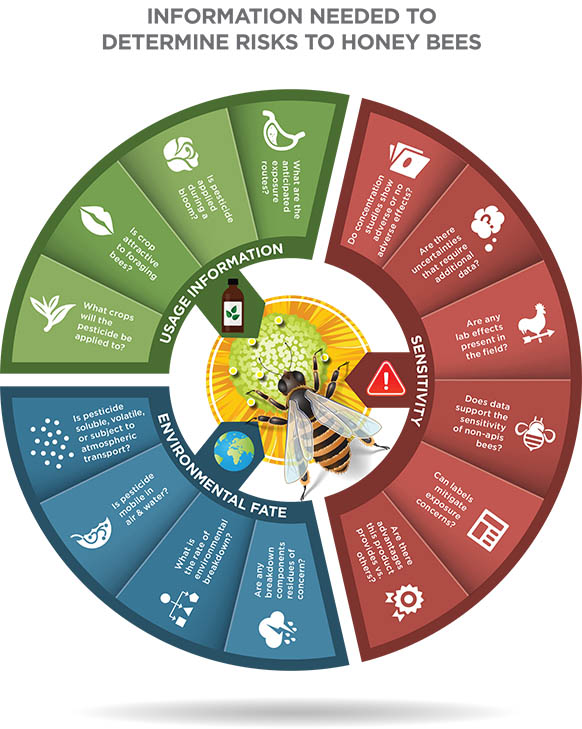
The bottom line is that FIFRA requires the EPA to answer the question: Does this product present an unreasonable risk to the environment when it is used under the conditions proposed for its use or that are currently in use?
To answer this question, the EPA needs to understand a product’s exposure and toxicity, and then needs to determine whether exposure is likely to harm each species. They will weigh the likelihood and magnitude of adverse ecological effects (that is, risk) against potential benefits.
During this process, the EPA also articulates a conceptual model of potential routes of exposure and effects. The EPA also identifies the overall plan for conducting the risk assessment.
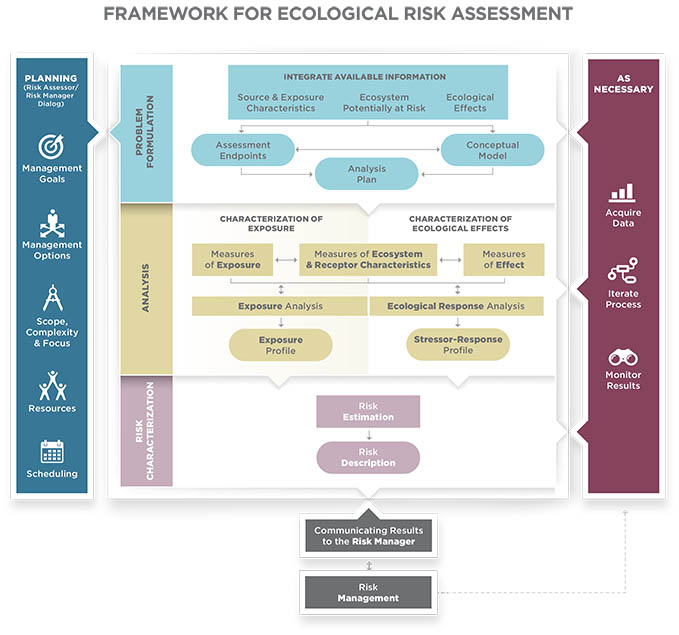
DETERMINING MANY ROUTES OF EXPOSURE
A key component of the risk assessment process is to identify a pesticide’s potential routes of exposure based on the product’s expected use. To evaluate the potential risks to bees, this is a rather complicated process, because investigators must delineate the number of ways that an individual honey bee and the colony can come into contact with the pesticide.
The figure on this page is an example of the various routes by which nontarget species can be exposed to pesticides. The information for the figure was taken from a recent assessment of a foliar-applied neonicotinoid systemic insecticide. The conceptual diagram depicted in the figure provides an example of:
• The pathways a systemic pesticide can be distributed within a plant
• How residues can then be transported to various matrices within the plant (such as pollen/nectar)
• And subsequently, how residues can be transported within the colony (through wax, royal and brood jelly) and expose the bees
The routes of exposure may be much simpler with nonsystemic pesticides. For any pesticide, exposure can result from direct application to the various environmental matrices (directly to bees, plants, soil, and water). However, the plant does not translocate nonsystemic pesticide residues into pollen and nectar.
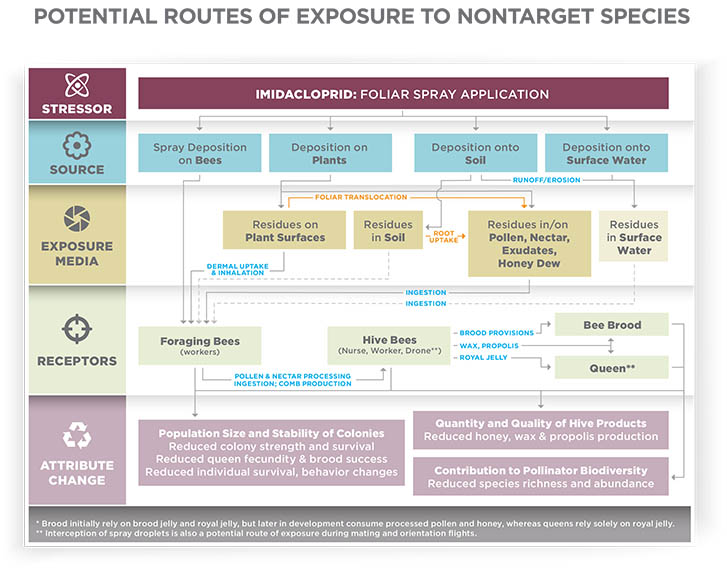
FORMULATING A WORKING HYPOTHESIS
After determining routes of exposure, the EPA creates a working hypothesis to determine how likely the pesticide could harm nontarget organisms such as honey bees. They base this risk hypothesis on the pesticide’s properties, possible routes of exposure, estimated environmental concentrations, and the measured toxicity of the pesticide. This initial risk hypothesis can (and often does) change as the review team becomes better acquainted with the pesticide’s toxicological and environmental characteristics.
ANALYZING THE DATA
The next phase of the risk assessment process includes an intensive review and summary of the available toxicity and environmental fate data. These data include registrant-submitted studies, studies published in scientific journals (open literature), environmental monitoring data, and ecological incident data.
The law requires that any studies a registrant submits to the EPA must indicate whether the studies followed Good Laboratory Practice (GLP) standards. The standards are meant to ensure consistency, reliability, and transparency in the data. Failing to comply with GLP standards can result in cancellations, suspensions, or modifications of a registration or research permit, penalties, or even criminal prosecution. Open literature studies must also meet the standard defined by the Data Quality Act. The EPA has published guidance for regulatory risk assessors on evaluating open literature studies for qualitative or quantitative use in ecological risk assessments that support regulatory decisions.
It should be noted that the EPA does not accept the data that registrants submit at face value. Registrants must submit raw data, methods, analyses, and conclusions to the EPA for reanalysis and re-review. After they receive a registrant’s study, the EPA re-enters the raw data, statistically reanalyzes the data, and generates an independent review and conclusion.
This process takes considerable time and resources, is subject to multiple levels of review within the agency, and is intended to be transparent so that the EPA’s understanding of the data is clear, concise, and reasonable.
In addition to formal laboratory- and field-basedtoxicity studies that registrants submit, the EPA also relies on monitoring data (pesticide residues in air, soil, and/or water) and incident reports about adverse effects (such as bird, fish and/or bee kills) to assess risk. Other regulatory authorities (including state and tribal governments), the registrants, or the general public provide these data.
Comparing Exposure and Effects in a Tiered Approach
To assess potential risks to pollinators, the EPA uses a process outlined in Guidance for Assessing Pesticide Risks to Bees (see References, page 68).
The EPA Risk Assessment Flowchart depicts the EPA’s tiered approach for assessing the risks from foliar-applied pesticides (we will describe the terms in the flowchart in more detail in the pages that follow). The illustration shows how the process moves from evaluating risk using laboratory-based studies of individual bees (Tier 1), to enclosure and/or feeding (semi-field) studies using whole colonies (Tier 2), to full-field studies using full-size colonies (Tier 3) under actual use conditions. The EPA uses similar flow diagrams (with slight differences) to evaluate the risk to honey bees from seed treatments and soil and tree applications.
A tiered approach allows the EPA to quickly evaluate (screen) pesticides that are shown not to harm pollinators. This allows them to allocate resources to address products likely to be of more concern. The EPA’s tiered approach to assess risks (and the tests underlying the process) are relatively consistent with those used in Europe and elsewhere. This reflects a common understanding of the science and is practical for the registrants who can submit the same data to other countries without having to generate new data.When a registrant develops a new product, the tiered approach provides them with an understanding of the criteria the EPA will use to evaluate the potential risks to bees from the proposed or existing uses of a pesticide.
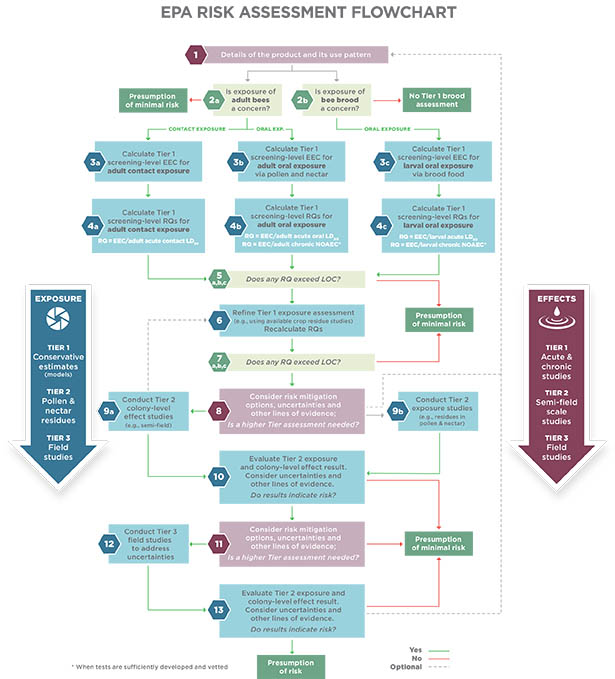
The EPA requires five Tier 1 laboratory-based studies on individual bees and larvae. The Tier 1 laboratory studies include acute tests (for single exposures) and chronic tests (for repeated exposures):
• Adult acute contact toxicity
• Adult acute oral toxicity
• Larval acute oral toxicity
• Adult chronic 10-day oral toxicity
• Larval chronic 21-day oral toxicity
If these studies indicate a cause for concern, then registrants may be required to complete a foliage residue toxicity study (a Tier 1 study).
Registrants may also be required to perform further studies that examine effects on the entire colony:
• Semi-field toxicity (Tier 2)
• Colony feeding toxicity (Tier 2)
• Full field toxicity (Tier 3)
Once a registrant generates the necessary data from the initial Tier 1 studies, they can readily calculate risk quotients to determine whether they need to test the product further. Registrants often weigh the potential costs associated with higher-tier testing versus what they expect the product will generate in the marketplace. In other words, if the expenses to test a pesticide in Tier 2 and Tier 3 studies is more than what a registrant expects to earn, they may choose not to register the product.
The Appendix (page 69) provides detailed descriptions of how researchers conduct all of these studies. We describe how the EPA uses the data from these studies below.
Tier 1 — Effects to Individual Honey Bees
The five required Tier 1 studies form a screening level assessment. The data from these studies help the EPA determine if the worst-case scenario of bee exposure in the environment would harm bees.
First, let’s look at what the Tier 1 studies measure, then let’s explore how the EPA uses those data measurements in their risk assessment process.
Acute and Chronic Studies
Remember that the EPA requires all registrants to submit five studies that examine the effects a pesticide will have on individual bees.
The primary goal of an acute toxicity study is to determine how much of a pesticide it will take to kill 50 percent of the individual bees they test. This standard, called the LD50 (for lethal dose, 50 percent), is a commonly used value in toxicology and has been found to be a reliable way to evaluate acute toxicity.
But the EPA testing guidelines for acute toxicity aren’t just concerned with bee death. The EPA specifies that toxicity studies must document all sublethal effects that may occur. If the data on sublethal effects are sufficient to support a reliable dose-response relationship, the EPA may use a median “effect dose” for 50 percent of the bees tested (called the ED50) to assess risk.
The primary goal of a chronic toxicity study is to determine a pesticide’s toxicity through repeated exposure. For chronic toxicity, researchers are trying to determine two values:
1. The no-observed adverse effect level (NOAEL) — this is the highest dose of the pesticide at which no adverse effects are observed
2. The lowest-observed adverse effect level (LOAEL) — this is the lowest dose of the pesticide that was observed to cause adverse effects
Chronic studies document sublethal effects on a bee’s growth, survival, and development. Sublethal effects for adult bees can include eating less, disproportionate agitation, excessive grooming, disorientation, and impaired foraging activity. Effects for larvae include decreased food consumption and increased time to pupation or adult emergence.
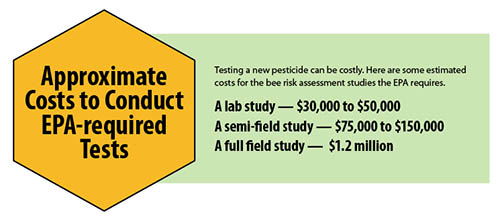
Estimated Environmental Concentrations, Risk Quotients, and Levels of Concern
So, the acute and chronic laboratory studies determine the level of pesticide exposure it will take to harm individual bees. But to fully estimate risk, the EPA also must know what the actual exposure might be.
To predict that exposure during the screening assessment, the EPA uses a model to determine the pesticide’s estimated environmental concentration (EEC). The EEC is a predicted concentration of the pesticide in the environment based on application rates, pesticide properties, and other factors. When the EPA sets this concentration, they use the worst-case scenario that predicts higher pesticide levels than would typically be found in the environment. After they determine the EEC, the EPA must convert the EEC to doses. They determine the doses by considering how much pollen or nectar bees of different castes, ages, and sexes may consume.
Now, the EPA can make a preliminary risk assessment by calculating a risk quotient (RQ).
The RQ is an equation that uses the dose (determined from the EEC) and one the measurements from the acute (LD50) and chronic (NOAEL) studies. That is, each Tier 1 study will have its own RQ — there will be a risk quotient for adult acute oral toxicity, another for adult acute contact toxicity, and so on. In addition, regulators calculate RQs for each crop and application method listed on the label. The risk is different for each scenario. In other words, a single pesticide may pose a low risk on some crops and a high risk on others.
To calculate the RQ, the EPA divides the EEC by the proper measurement from the studies. That is, each Tier study will have its own RQ — there will be risk quotient for each route of exposure. For example, the RQ for adult acute oral toxicity would be:
Exposure (e.g., dose) ÷ LD50 = RQ
The exposure (e.g., dose) is determined by the EEC, and (in this example) the LD50 comes from the adult acute oral toxicity study.
After the EPA determines the RQ, they compare that number to the level of concern (LOC). For pesticides, the LOC for acute risk is always 0.4 and the LOC for chronic risk is always 1.0.
Let’s consider a simple example. Let’s say that an adult acute oral toxicity study determined that the LD50 for a product was 100 parts per million (ppm). The dose (which predicts worst-case exposure) for the product was 15 ppm. So:
15 ÷ 100 = 0.15
For this example, the product would have a risk quotient of 0.15, which puts it well below the 0.4 level of concern.
Screening Risk
The EPA Risk Assessment Flowchart (page 55) shows us an example of what the EPA would do at this stage. The EPA compares the RQ values and the LOC values. If the RQ values are lower than the LOC values, then the EPA presumes that the product will post a minimal risk to the health of the colony.
If RQ values are greater than the LOC values, that doesn’t automatically mean that there will be an unacceptable risk in the environment. Remember that the EPA predicts the EEC (environmental exposure) based on worst-case estimates.

The EPA can refine its overall risk assessment by using actual measured pesticide residue levels on pollen, nectar, and foliage rather than the predicted EECs (when such data are available). These actual measured levels come from submitted studies. Researchers typically conduct such studies on multiple crops and, depending on the crop, researchers collect samples for residue analyses directly from the treated plants or from bees foraging on the treated crop.
If a foliage toxicity study shows that the actual environmental exposure is significantly lower than the predicted EEC, then the EPA can use this actual level (instead of the EEC) to refine the RQ.
If the RQ calculated with the actual exposure falls below the LOC, then the EPA presumes that the product will pose a minimal risk to individual bees (and by extension, to the colony). At this point, the pesticide may pass the Tier 1 screen as long as no other data (open literature studies, incident data) indicate potential adverse effects.
Even if the RQ with the actual exposure is still greater than the LOC, the registrant can work with the EPA to develop mitigation measures. However, the EPA has to agree to any proposed risk mitigation measures intended to reduce exposure.
These mitigation measures could include one or more of the following:
• Prohibiting applications to blooming crops
• Limiting applications to when bees are less likely to be foraging (dusk to dawn)
• Reducing application rates
• Reducing the number of applications
• Increasing the time between applications (longer reapplication intervals)
• Restricting use to crops with lower exposure levels
• Limiting the type of application equipment
While these are legitimate ways to reduce exposure to honey bees, these tactics can also reduce the product’s efficacy and economic benefits, so they are not always feasible. Some insect pests have multiple life cycles or the pest pressure might be high enough to warrant multiple applications. So, reducing a pesticide rate might effectively reduce how much pesticide is being applied, it might also mean that it no longer effectively controls the pest. Reduced rates may also lead to target insects developing pesticide resistance.
Depending on the EPA’s need for additional information, they may require Tier 2 and possibly Tier 3 studies to address unanswered questions.
TIERS 2 AND 3 — COLONY-LEVEL ASSESSMENTS
While pesticide exposure can harm bees at the individual level, it is the pesticide’s overall impact to the colony that is critical to the health and survival of social bees. Given the complexities of a honey bee colony and its division of labor, and the fact that honey bees may forage on a variety of floral sources rather than exclusively on a single crop, the colony may be able to tolerate higher levels of a pesticide exposure than individual bees under laboratory test conditions.
However, risk assessors do not use data from colony-level studies to calculate RQ values. While there are tools to estimate exposure to individual bees, it is more complicated to estimate exposure to the whole colony.
Currently, the data collected during Tier 2 and Tier 3 studies are evaluated in combination with laboratory studies of individual bees (from both registrant-submitted studies and those gleaned from the open literature). These evaluations help determine whether there is enough information to refute or support the risk hypothesis associated with a particular pesticide.

The EPA’s understanding of the risk assessment process continues to evolve as the science evolves. The EPA has identified additional studies to assist in the risk assessment process. For honey bees, they have added multiple studies to help quantitatively and qualitatively assess risks. Although many of the studies are not currently specified in the Code of Federal Regulations Part 158, the EPA can require such studies as it deems necessary to support risk assessments and decision-making.
The EPA has worked closely with stakeholders to ensure that they are aware of the data needed to support the risk assessment process. In many cases, registrants submit the data packages to the EPA with the full complement of studies, particularly in cases where a compound is proposed for use at bloom on crops that are attractive to pollinators.


The EPA has been working with researchers in the USDA-Agricultural Research Service to develop a colony simulation model to estimate the effects of pesticides on honey bee colonies. The model uses laboratory toxicity data about pesticides to simulate the effects on colony condition (numbers of adult and brood bees) and queen performance. The model can also look at the interaction of pesticide exposure with Varroa mite loads.
Such predictive tools (after being sufficiently vetted) can serve as another line of evidence to support risk assessments and may reduce the need for more elaborate colony-level studies. Other regulatory agencies around the world are developing similar models. So, the EPA (and its international counterparts) continue to advance the science of assessing risks to bees.
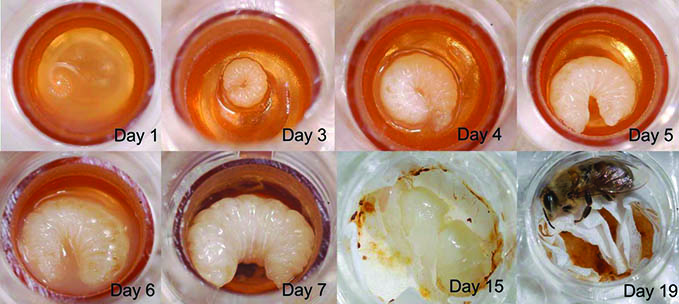
IS THE RISK QUOTIENT ABOVE OR BELOW LEVELS OF CONCERN?
The “last” phase of the risk assessment process we discuss is risk characterization. This phase consists of two parts: risk estimation and risk discussion. The EPA generally quantifies risk as a ratio between expected environmental concentrations and the most sensitive acute and chronic toxicity values for nontarget organisms.
The EPA uses a model to estimate a pesticide’s exposure to bees via contact and diet (pollen and nectar consumption) for various ages and classes of bees. The model uses worst-case situations that predict higher pesticide levels than would typically be found in the environment.
For the screening-level assessment, the EPA focuses on determining if a pesticide is unlikely to harm bees even under the worst-case scenario. The concept is simple enough: The pesticide can harm bees if the level of environmental exposure is greater than the level shown to be toxic. If the amount of pesticide in the environment (even at its worst-case level) is sufficiently lower than the toxic level (determined from laboratory studies), then regulators predict that bees are unlikely to be adversely affected by contact exposure or by ingesting nectar and pollen from peak residue levels in the treated crop.
For pesticides that exceed levels of concern, regulators can refine the risk assessment based on higher-tier effects data and exposure data (measured residues in pollen and nectar). For these assessments, regulators often determine the risk for the whole colony (from the Tier 2 and Tier 3 studies) as well as individual bees.

Registration Decisions Go Through Extensive Reviews
The EPA’s decision whether to register or reregister an active ingredient considers the products potential effects on human health and wildlife, coupled with an understanding of the benefits that the compound affords. A division of the EPA evaluates the benefits of using a compound. This is an ongoing process for chemicalsalready registered. The law requires the EPA to re-evaluate each chemical on a 15-year cycle to ensure that the underlying data and assessment methods are current with the science.
FIFRA requires the EPA to determine whether a compound meets the FIFRA “standard” — that using a compound does not represent an unreasonable risk to human health and the environment. The EPA may determine that a pesticide is ineligible for registration or reregistration if the risks exceed the benefits the product provides. If the benefits outweigh risks, then the EPA may proceed with registration/reregistration and require the registrant to place risk reduction measures on the label.
The Federal Register publishes the outcome of the risk assessment and risk management decision. The public can provide written comments directly to the agency about the the science used and the conclusions drawn from the risk assessment. Stakeholders (registrants, advocacy groups, the public) are free to pursue legal measures if they disagree with the regulatory decision.
The Label Puts the Risk Assessment to the Test
The label forms the foundation for the risk assessment process and ensures that the compound is used in a way that is consistent with the FIFRA standard. Users must follow Directions for Use and advisory hazard statements on product labels to avoid posing an unreasonable risk to human health or the environment.
The EPA approves all pesticide labels as part of the registration and reregistration process. The EPA may require specific instructions appear under the environmental hazard statement to protect honey bees and pollinators.
The EPA requires a pesticide label to carry advisories/warnings when the product may contaminate various habitats or harm particular taxa. These statements are typically based on the environmental fate and toxicity data evaluated during the risk assessment process.
Examples of standard hazard statements include:
• “This pesticide is toxic to bees exposed to direct application. Applications should be timed to coincide with periods of minimum bee activity, usually late evening and early morning.”
• “This product is highly toxic to bees exposed to direct treatment or residues on blooming crops or weeds. Do not apply this product or allow it to
drift to blooming crops if bees are visiting the treatment area.”
In order to achieve more consistent label language, the EPA developed the Label Review Manual (see References, page 68) to guide risk managers. The manual relies on data submitted in support of registration and reregistration. However, pesticides can differ widely in their environmental fate and effects. The EPA has the option to add more restrictive language to the label. Such language allows the EPA to tailor a pesticide label to be prescriptive and to provide additional instructions that allow for case-by-case mitigation measures to fine tune how the product is used.

MOVING BEYOND THE LABEL
While pesticide labels are intended to help minimize the risks to human health and the environment, stakeholders can perform voluntary actions to further reduce exposing bees to pesticides.
The EPA has been working with state/tribal lead agencies and a broad range of stakeholders to develop managed pollinator protection plans (or MP3). In most cases, the MP3s involved voluntary collaborations between stakeholders. For example, some of the plans have encouraged beekeepers to voluntarily register their colonies so that growers and applicators have a way to know where apiaries are located.
The National Strategy to Promote the Health of Honey Bees and Other Pollinators (see References, page 68) has echoed the importance of communication, cooperation, and collaboration between a broad range of stakeholders. They also encourage public-private partnerships that can further our understanding of factors that affect pollinators and to develop ways to mitigate those factors. The National Strategy also identifies what research we need to advance our understanding of factors affecting honey bees and other pollinating species.

CONCLUSION
While big, powerful, beautiful, and rare animal species often dominate our attention, the common honey bee is no less a marvel. Those who work in agriculture understand that a healthy, productive, profitable, and sustainable farming operation depends largely on these and other unseen organisms. So, it is important that we understand them and take steps to minimize the risks we expose them to.
Most farmers aim to do as little harm as possible to pollinators while growing crops sustainably and economically. It doesn’t matter if the farm is small or large, organic or traditional, pollinators play a pivotal role in boosting crop yields for many of the vegetables and fruits we consume each and every day.
However, pollinators represent more than just the food we enjoy. Unlike many of the wildlife species we see on nature programs, we can observe insect pollinators in our backyards. We can easily watch honey bees, butterflies, and other native pollinators moving from flower to flower in our gardens and landscapes.
Pesticides are part of our agricultural system, so the challenge before us is to minimize their exposure to pollinators. Let’s return to the question we posed at the start of this document: “Are honey bees in decline?”
Managed honey bees certainly face multiple challenges. While USDA data indicate that the total number of managed colonies has been relatively constant in the United States since the mid-1990s, the data do not reflect the extra effort beekeepers have made to maintain these numbers.

In national surveys, beekeepers consistently report that they can compensate for losses of between 15 to 17 percent; however, overwintering losses the past decade have averaged 31 percent, and more recent estimates of total annual losses have indicated a loss rate of 42 percent. Both estimates are well above what beekeepers have indicated as economically sustainable.
We have focused on the relatively unique biology of the honey bee, how a honey bee colony functions as a superorganism, and how regulators use this information to evaluate the potential role of one risk factor, pesticides. The risk from any pesticide is a complicated function of exposure and toxicity which vary depending on the biological cycles of the pollinator species. Clearly, there are chemicals classified as highly toxic to bees, and sufficient exposure can harm individual bees and the colony.
Pesticide applicators and beekeepers are key components of our food production system. If they work together, they can achieve their individual goals and their mutual goal of maintaining an abundant food supply. Increasing communication between stakeholder groups will help to reduce misinformation and may provide an opportunity for greater collaboration and cooperation between growers, applicators, and beekeepers. In turn, communication can reduce pesticide exposure and risk to bees.
Although pesticides are one of multiple factors affecting bees, increasing awareness and mitigating the effects of each factor will help to reduce the cumulative impact. Although we focused on honey bees in this publication, the considerations and protections we describe will extend to other non-Apis bees and beneficial insects that provide ecosystem services for our shared environment.

References
Guidance for Identifying, Selecting and Evaluating Open Literature Studies (EPA)
www.epa.gov/pesticide-science-and-assessing-pesticide-risks/guidance-identifying-selecting-and-evaluating-open
Framework for Ecological Risk Assessment (EPA)
www.epa.gov/sites/production/files/2014-11/documents/framework_eco_assessment.pdf
Good Laboratory Practice Standards Compliance Monitoring Program (EPA)
www.epa.gov/compliance/good-laboratory-practices-standards-compliance-monitoring-program
Guidance for Assessing Pesticide Risks to Bees
www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf
Label Review Manual (EPA)
www.epa.gov/pesticide-registration/label-review-manual
National Strategy to Promote the Health of Honey Bees and Other
Pollinators
obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/Pollinator%20Health%20Strategy%202015.pdf
Series 850 — Ecological Effects Test Guidelines (EPA publications)
www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-850-ecological-effects-test-guidelines
USDA Honey Bee Surveys and Reports
www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Bee_and_Honey
White Paper in Support of the Proposed Risk Assessment Process for Bees
www.cdpr.ca.gov/docs/emon/surfwtr/presentations/epa_whitepaper.pdf
Indiana’s MP3
www.oisc.purdue.edu/pesticide/p3_activities.html
The EPA requires five tier 1 laboratory-based studies on individual bees and larvae.
The Tier 1 laboratory studies include acute tests (for single exposures) and chronic tests (for repeated exposures):
• Adult acute contact toxicity
• Adult acute oral toxicity
• Larval acute oral toxicity
• Adult chronic 10-day oral toxicity
• Larval chronic 21-day oral toxicity
In addition to these tier 1 studies, the EPA may require these tests:
• Foliage residue toxicity (a Tier 1 study)
• Semi-field toxicity (Tier 2)
• Colony feeding toxicity (Tier 2)
• Full field toxicity (Tier 3)
Laboratory studies typically use the pure active ingredient (referred to as technical grade active ingredient, TGAI). That said, the EPA may require studies to be conducted using the technical end product (TEP), which is the formulated product available to applicators or consumers. The TEP contains the active ingredient(s) plus other inert ingredients that can influence how target and nontarget organisms absorb and distribute the active ingredient.
This appendix describes each of the methods and protocols for these tests.
Required Tests
Adult Acute Contact Toxicity
The adult acute contact toxicity test (OCSPP 850.3020; OECD Test Guideline 214), is a 48-hour test that typically uses 25 to 30 adult bees for each of five dose levels.
Bees in each dose level may be divided into replicates.
For this test, researchers briefly anesthetize young adult bees and place a single dose of the test material in a suitable solvent (typically acetone) on the back (top thorax) of the adult honey bee. Researchers must also run suitable experiment control groups at the same time. Control groups include running the test with water alone (referred to as the negative control), running the test with the solvent (if used), and running the test with a known toxicant (typically the organophosphate insecticide dimethoate) referred to as the toxic reference (or positive) control.
Researchers record signs of toxicity (number of dead and disabled) at four, 24, and 48 hours. If adult bee mortality continues to increase at 48 hours, researchers extend the observation period to 96 hours. One outcome of this study is if the data indicate that less than 11 micrograms (μg) per bee is toxic, then a follow-up toxicity of residues on foliage test may be required.
Adult Acute Oral Toxicity
The adult acute oral toxicity test (OECD Test Guideline 213) involves testing five different dose levels. For each level, there are three different groups (replicates) of at least 10 honey bees.
For this test, researchers feed bees 100 to 200 microliters (µL) of 50 percent sucrose solution that they spiked with the various dose levels of the pesticide. After a maximum of six hours, researchers replace the treatment solution with an unspiked sucrose solution. Researchers then determine how much of a dose the bees consumed by measuring how much of the spiked solution remains.

Researchers observe the bees and record mortality and sublethal effects at four, 24, and 48 hours. If adult bee mortality continues to rise by more than 10 percent after the first 24 hours, they extend the test duration to 96 hours. In addition to the five pesticide dose levels, researchers must include suitable controls (negative; solvent if warranted; and, reference).
Larval Acute Oral Toxicity
The larval acute oral toxicity test (OECD Test Guideline 237) uses first instar bee larvae.
Researchers place the larvae into separate wells of 36- or 48-well plates and feed them royal jelly combined with several sugars (carbohydrate source) and yeast extract (protein source). The diet is intended to be similar to what nurse bees feed to developing larvae in honey bee colonies. As with the oral acute oral test, researchers test five different dose levels for the pesticide and include replicates. Typically, researchers use a minimum of 12 larvae collected from three separate honey bee colonies for each replicate.
For the first three days of the study, researchers feed larvae an untreated diet. On day four, researchers provide a single dose of spiked diet (with a known concentration of test material). Typically, larvae consume the entire amount of spiked diet within the day. After day four, researchers resume feeding bees the untreated diet.
As with the other toxicity tests, researchers record mortality and sublethal effects. They measure on days five, six, and seven of the test and then calculate the 72-hour LD50 (cumulative mortality at day seven). As with the acute toxicity tests with adult bees, researchers run a negative control (untreated diet), a solvent control (if used to dissolve the test item), and a positive control (typically the insecticide dimethoate and/or the insect growth regulator fenoxycarb).
Adult Chronic 10-day Oral Toxicity
The adult chronic oral toxicity test (draft OECD Test Guideline) is based on for the adult acute oral toxicity test. Again, researchers test five doses with three replicates at each dose. Each replicate typically has a minimum of 10 young (maximum of two-days old) adult honey bees. Researchers expose honey bees to a 50 percent (weight to volume) sugar solution that contains a dose of the test chemical over a period of 10 days. Researchers evaluate the effects of the chemical by comparing effects in the treated group to those of the respective control. Researchers focus mainly on mortality, but they also must report sublethal effects (such as impaired behavior, decreased/increased food consumption).
Researchers then determine the 10-day no-observed effect concentration (NOAEC based on concentration in the diet), the 10-day NOAEL (based on dose), the median lethal concentration for 50 percent of the bees (10-day LC50 based on concentration in the diet), and the 10-day LD50 (based on dose). Depending on the quality of the dose-response relationship, researchers may use a measurement endpoint other than mortality to calculate a 10-day EC50 and/or 10-day ED50. Similar to the acute toxicity tests, researchers run negative and solvent controls, and reference toxicant control (typically dimethoate).

Larval Chronic 21-day Oral Toxicity
The larval chronic oral toxicity test (OECD Guidance Document 239) is based on the design of acute larval toxicity test. Again, researchers collect a minimum of 12 first instar larvae collected from three separate honey bee colonies. Each set of 12 larvae are a replicate, and the researchers have three replicates for five treatment levels.
Initially, researchers feed larvae an untreated diet. From day three through day six of the study, researchers provide a spiked diet that contains an increasing known concentration of test material — larvae are repeatedly dosed for four consecutive days. Larvae stop eating after this time to make the transition to a prepupa.
The study continues until the adult bees emerge at day 22. Researchers record mortality and other abnormal effects daily from days three through day eight, and then again on day 15. Researchers also record the cumulative number of emerged bees on day 22. Researchers determine the NOAEC (based on concentration in the diet), NOAEL (based on dose), the median effect concentration for 50 percent of the bees (EC50 based on concentration in the diet), and the ED50 (based on dose) are determined for adult emergence on day 22. The study can also provide a LC50, LD50, and NOAEC/NOAEL for larval survival at day eight. As with the other tests, researchers also run negative and solvent controls and a reference toxicant control (typically dimethoate or fenoxycarb).
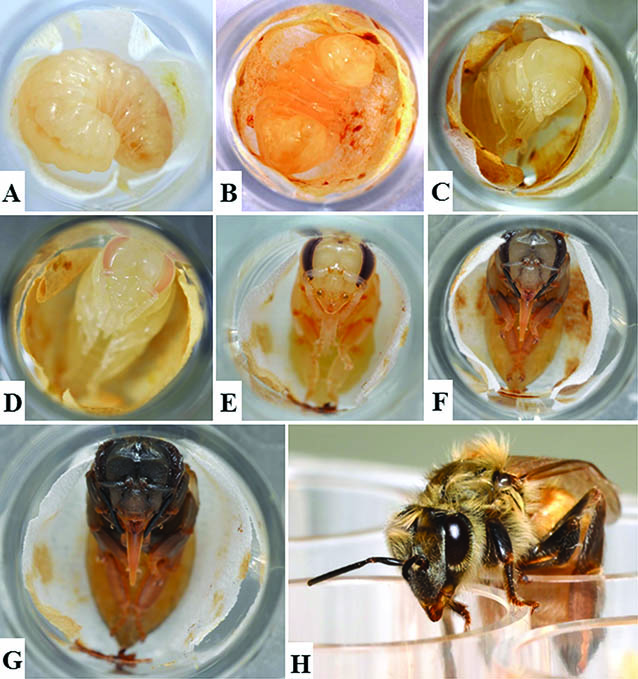 These images show a honey bee transition (metamorphosis) from prepupa (A) to emergence (eclosion) as an adult (H). © The International Bee Research Association. Reproduced with permission of the editors of the Journal of Apiculturual Research.
These images show a honey bee transition (metamorphosis) from prepupa (A) to emergence (eclosion) as an adult (H). © The International Bee Research Association. Reproduced with permission of the editors of the Journal of Apiculturual Research. 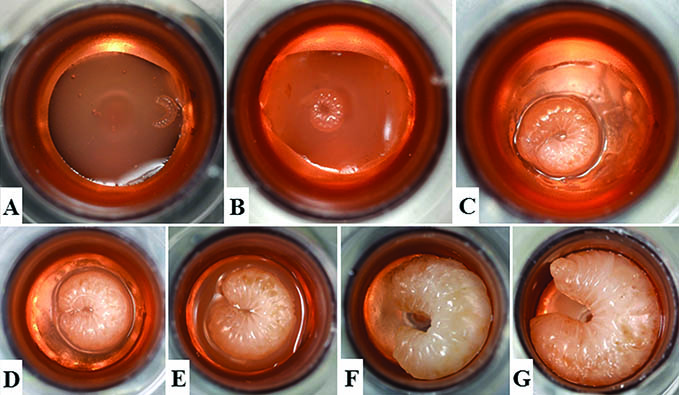 These photos show a larval toxicity test. Each well contains a larva at a different stage of development from first instar (newly hatched) larva (A) to prepupa (G). The larvae are surrounded by brood jelly. Larvae stop eating when they transition to prepupa. © The International Bee Research Association. Reproduced with permission of the editors of the Journal of Apiculturual Research.
These photos show a larval toxicity test. Each well contains a larva at a different stage of development from first instar (newly hatched) larva (A) to prepupa (G). The larvae are surrounded by brood jelly. Larvae stop eating when they transition to prepupa. © The International Bee Research Association. Reproduced with permission of the editors of the Journal of Apiculturual Research. Tests that May Be Required
Foliage Residue Toxicity
The foliage residue toxicity test (OCSPP 850.3030) is considered a Tier 1 study, but it is only required for pesticides that are moderately or highly toxic (LD50<11 µg/bee) via acute contact exposure. This study determines the length of time that a compound remains toxic to bees exposed to the treated foliage.
Researchers use the study to calculate the RT25, which is defined as the residual time needed to reduce the activity (toxicity) of the test substance and bring bee mortality down to 25 percent of the tested populations.
The study has a field component in which researchers make foliar applications to alfalfa and then treated foliage/residues are weathered for various amounts of time. For the laboratory component of this study, researchers expose bees to the freshly cut weathered foliage from different time points. While the RT25 is not used in calculating risk, the data have been used to develop label advisory statements that include the length of time a test substance is expected to remain toxic to bees following contact exposure to residues of the pesticide on vegetation.
Some beekeepers and growers consider the RT25 to be a useful way to identify products that are less hazardous to bees, particularly if they need to apply the pesticide at a specified time (such as before bees are likely to be foraging). For example, if the RT25 is less than three hours, applicators might apply the product after dusk with some certainty that it is not likely to represent a hazard to bees foraging on that crop the following day.
However, the RT25 is like other data used in evaluating risk to bees — it represents one of several lines of evidence.
The EPA may also require field testing on whole colonies. The EPA may request such studies if Tier 1 studies indicate that a compound is toxic to bees and initial exposure estimates suggest that environmental concentrations may be sufficient to result in adverse effects on individual bees.
As studies transition out of the laboratory and into more realistic exposure scenarios using entire bee colonies, then researchers typically use the actual product that growers and applicators will actually use — as opposed to just using the active ingredient in the lab. As such, the semi-field (Tier 2) and full-field (Tier 3) studies include both the active ingredient and the “inerts” as part of the formulated product. Transitioning to less controlled environments, means that researchers must contend with weather, pests, diseases, and poor nutrition that can confound test results.
Semi-field Toxicity
The semi-field toxicity test (Tier 2 Tunnel Study, OECD 75 and EPPO 170) is a Tier 2 study that uses tented enclosures and small nucleus colonies (typically five-frame colonies with approximately 5,000 to 7,000 bees). During this study, bees must forage in confined areas that have been treated or where bees are provided spiked diets (treated sucrose solution and/or spiked pollen and protein).
In semi-field enclosure studies, researchers typically apply the maximum pesticide rate on a pollinator-attractive crop (buckwheat, alfalfa, phacelia). This rate ensures maximum exposure, and the study includes a toxic reference (typically dimethoate and/or fenoxycarb) and suitable negative controls to ensure that the study is sensitive.
In a number of the studies, researchers apply the compound by ground equipment while bees are actively foraging to further ensure maximum “worst case” exposure. Since enclosures are somewhat limited in area (about 200 feet x 330 feet), bees can undergo confinement stress in such studies. For this reason, researchers do not typically keep colonies in the enclosures any more than two weeks. However, researchers continue to monitor exposed colonies after removing them from the enclosures to determine whether there are any long-term effects on the colonies. These monitoring periods typically include at least one full brood cycle (21-days), but frequently include multiple brood cycles. Bees are removed to a clean site, preferably away from agricultural fields to limit exposure to other pesticide products during monitoring.
Colony Feeding Toxicity
The colony feeding toxicity test (a Tier 2 study) typically uses a range of exposure levels to determine a NOAEC and LOAEC for adults and brood.
Researchers can feed bees spiked diets for short intervals (one feeding) to multiple feeding periods (like six weeks). The extended periods simulate longer duration exposures that may occur in crops with prolonged bloom periods, such as with indeterminate blooming crops (cotton, squash, strawberries). Since the more extensive feeding studies typically last longer and frequently involve larger colonies, the colonies are monitored for longer periods. Some studies may even include an overwinter component.
While not intended to be exhaustive, here are some examples of measurements that researchers can collect at the whole colony level:
• Forager bee activity and mortality
• Queen fecundity (egg production)
• Brood development and survival
• Hive weight and strength (adult and larval bee abundance)
• Quantity and quality of food reserves in hive
Full-field Toxicity
The full-field toxicity test (Tier 3 Study; OCSPP 850.3040) is a Tier 3 study. The EPA tries to ensure that the study design builds on existing data and focuses on remaining uncertainties. Tier 3 studies are intended to answer very specific questions and the design depends on the risk hypothesis. Researchers may study full-size colonies (approximately 20,000 to 40,000 bees) with free-foraging bees are and exposure conditions are designed to be as close to actual pesticide use conditions as possible.
Since the EPA Office of Chemical Safety and Pollution Prevention does not have formal, detailed guidelines for Tier 2 or Tier 3 studies with honey bee colonies, the EPA typically requires registrants to submit proposed protocols for EPA review before initiating the study. For both the Tier 2 and Tier 3 studies, the EPA is particularly interested in whether the colony is able to recover from what may be direct (but often transient) effects on survival, growth, or development of the colony.

ACKNOWLEDGEMENTS
Thanks to Dawn Minns for graphic design and Adduci Studios for illustrations. Thanks also to those who offered constructive comments during the development of this publication:
Silvia Hinarejos, Valent USA
Tammy Horn, Kentucky Department of Agriculture
Melvin Nicholson, Nicholson Consulting Service
Dan O’Hanion, Beekeeper
Bridget O’Neill, Dupont
Don Parker, National Cotton Council
Ken Simpson, Beekeeper
Disclaimer
This publication is intended for educational purposes only. The authors’ views have not been approved by any government agency, business, or individual and cannot be construed as representing a perspective other than that of the authors. The publication is distributed with the understanding that the authors are not rendering legal or other professional advice to the reader, and that the information contained herein should not be regarded or relied upon as a substitute for professional consultation. The use of information contained herein constitutes an agreement to hold the authors, companies or reviewers harmless for liability, damage, or expense incurred as a result of reference to or reliance upon the information provided. Mention of a proprietary product or service does not constitute an endorsement by the authors or their employers. Descriptions of specific situations are included only as hypothetical case studies to assist readers of this publication, and are not intended to represent any actual person, business entity or situation. Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind by Purdue University. Individuals using such products assume responsibility that the product is used in a way intended by the manufacturer and misuse is neither endorsed nor condoned by the authors nor the manufacturer.
This publication is supported by the Office of Indiana State Chemist Pollinator Protection Plan. More information about the plan is available on the state chemist’s website: www.oisc.purdue.edu/pesticide/p3_activities.html.