Discovery reveals how a specialized structure in plant cells helps regulate photosynthesis
More insight into the process could improve crop productivity
WEST LAFAYETTE, Ind. — Purdue University scientists have discovered a key mechanism that regulates how plants develop chloroplasts, essential structures responsible for the photosynthesis that sustains life on Earth by producing oxygen and food.
Chloroplasts are semi-autonomous organelles that have some degree of independence in their operation and reproduction. However, they still require proteins made from instructions in the cell nucleus to function properly and to assemble multiprotein complexes like the photosynthetic apparatus. Specialized transporters in the outer chloroplast membrane must convey these proteins into the chloroplast.
A new paper in Science Advances by Gyeong Mee Yoon, associate professor of botany and plant pathology, and Yuan-Chi Chien, PhD student, describes how plants control this import process by regulating the stability of transporter called the TOC (translocon at outer envelope of chloroplasts) complex. Yoon and Chien identified a crucial amino acid of the transporter that, when modified, acts as a molecular switch governing these transporters.
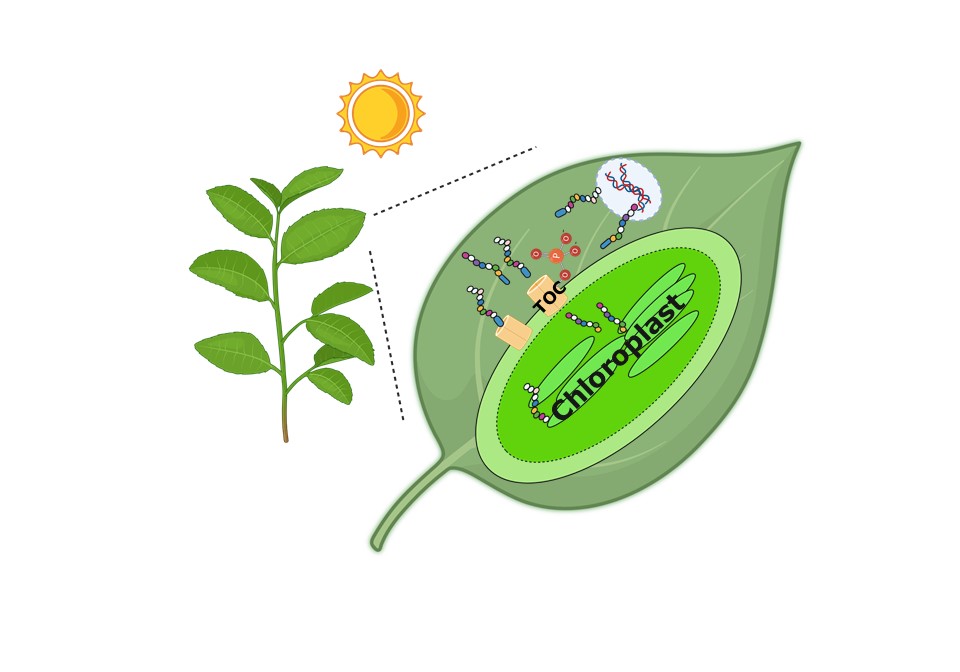 The molecular machinery of the chloroplast regulates how efficiently plants convert sunlight into energy through photosynthesis. Purdue University scientists have discovered a new mechanism that controls plant chloroplast development. To properly function, chloroplasts must import chloroplast-destined proteins through a gateway called the TOC complex. The Yoon lab found that a chemical modification called phosphorylation (addition of PO₄³⁻) to these gateway proteins enhances this process by extending their lifespan, allowing more proteins to be efficiently imported into the chloroplasts. (Image provided by Gyeong Mee Yoon, Purdue University).
The molecular machinery of the chloroplast regulates how efficiently plants convert sunlight into energy through photosynthesis. Purdue University scientists have discovered a new mechanism that controls plant chloroplast development. To properly function, chloroplasts must import chloroplast-destined proteins through a gateway called the TOC complex. The Yoon lab found that a chemical modification called phosphorylation (addition of PO₄³⁻) to these gateway proteins enhances this process by extending their lifespan, allowing more proteins to be efficiently imported into the chloroplasts. (Image provided by Gyeong Mee Yoon, Purdue University). Altering this amino acid and the chemical modification can dramatically disrupt chloroplast development and structure, said Yoon, who also is a member of the Purdue Center for Plant Biology. “This discovery deepens our understanding of plant biology and opens promising avenues for improving crop yields since chloroplasts are fundamental to plant productivity.”
Their work entailed sorting through and genetically manipulating the functions of a complicated alphabet soup of plant proteins in controlled experiments using a model plant Arabidopsis thaliana. The TOC33 protein lies at the heart of the study. This protein is a component of the larger TOC complexes operated in the chloroplast outer membrane. It recognizes and facilitates the entry of chloroplast-targeted proteins to the chloroplast during early plant development.
The process that regulates the TOC protein function remains elusive. Through this process, Yoon said, “the plant can take in the necessary chloroplast-targeted proteins selectively.”
Yoon’s lab specializes in the biology of ethylene, a gaseous plant hormone that plays a key role in responding to environmental stress and fruit ripening. As Yoon and Chien noted in their paper, “ethylene plays a profound role in chloroplast function and photosynthesis.”
A well-known kinase — a molecular on-off switch — called CTR1 (Constitutive Triple Response 1), regulates ethylene signaling. When activated, it inhibits ethylene signaling; when deactivated, it promotes the signaling. Surprisingly, Yoon said, the function of CTR1 in regulating TOC function is an ethylene-independent process.
“The CTR1 kinase happens to be localized in the outer membrane of the chloroplast, which is unexpected,” she said. This is another new finding because scientists have previously found CTR1 only within a network of cellular membranes called the endoplasmic reticulum, with some translocation to the nucleus under stress conditions.
CTR1’s main job in the chloroplast is to phosphorylate the TOC33 at a specific location — at position 260 in the protein chain of an amino acid called serine. By altering this site in the plants, Yoon and Chien confirmed that the CTR1 kinase phosphorylated TOC33. This chemical modification at serine 260 helps stabilize TOC33, thus enhancing the import of chloroplast-targeted proteins and thereby controlling chloroplast development.
A stable TOC complex makes more of its proteins available early in chloroplast development. Reducing the flow of these proteins impairs chloroplast development, resulting in poor plant growth or reduced crop yields. “When the TOC33 protein cannot be modified at serine 260, it becomes less stable than the natural version. This shows that this specific spot is like a switch that helps keep the protein stable and functioning properly,” Yoon said.
Plant biologists can engineer this process in the laboratory. “Our research demonstrates that we can influence chloroplast biogenesis and development, which opens up promising avenues to potentially enhance crop yields and food security in the future,” she said.
This research was supported by the National Science Foundation.
About Purdue Agriculture
Purdue University’s College of Agriculture is one of the world’s leading colleges of agricultural, food, life and natural resource sciences. The college is committed to preparing students to make a difference in whatever careers they pursue; stretching the frontiers of science to discover solutions to some of our most pressing global, regional and local challenges; and, through Purdue Extension and other engagement programs, educating the people of Indiana, the nation and the world to improve their lives and livelihoods. To learn more about Purdue Agriculture, visit this site.
About Purdue University
Purdue University is a public research university leading with excellence at scale. Ranked among top 10 public universities in the United States, Purdue discovers, disseminates and deploys knowledge with a quality and at a scale second to none. More than 106,000 students study at Purdue across multiple campuses, locations and modalities, including more than 57,000 at our main campus locations in West Lafayette and Indianapolis. Committed to affordability and accessibility, Purdue’s main campus has frozen tuition 14 years in a row. See how Purdue never stops in the persistent pursuit of the next giant leap — including its integrated, comprehensive Indianapolis urban expansion; the Mitch Daniels School of Business; Purdue Computes; and the One Health initiative — at https://www.purdue.edu/president/strategic-initiatives.
Writer: Steve Koppes
Media contact: Devyn Ashlea Raver, draver@purdue.edu
Sources: Gyeong Mee Yoon, yoong@purdue.edu
Agricultural Communications: Maureen Manier, mmanier@purdue.edu, 765-494-8415
Journalist Assets: Publication quality charts and photos are available at this link