The Keys to Unlocking Plant Reproductive Behavior
If you took a biology class in high school, you probably learned that flowering plants reproduce when sperm cells, carried by pollen, fertilize an egg in the ovule of the plant. But how does the pollen get to the ovule, and how does it know when to release the sperm?
“It’s important that the process works correctly so you get seeds, and the plant needs to control what kind  of pollen it allows so that it’s not wasting resources,” says Sharon Kessler, associate professor of botany and plant pathology. “But this way of communicating is not only happening during reproduction, it’s also happening during normal plant growth and development processes.”
of pollen it allows so that it’s not wasting resources,” says Sharon Kessler, associate professor of botany and plant pathology. “But this way of communicating is not only happening during reproduction, it’s also happening during normal plant growth and development processes.”
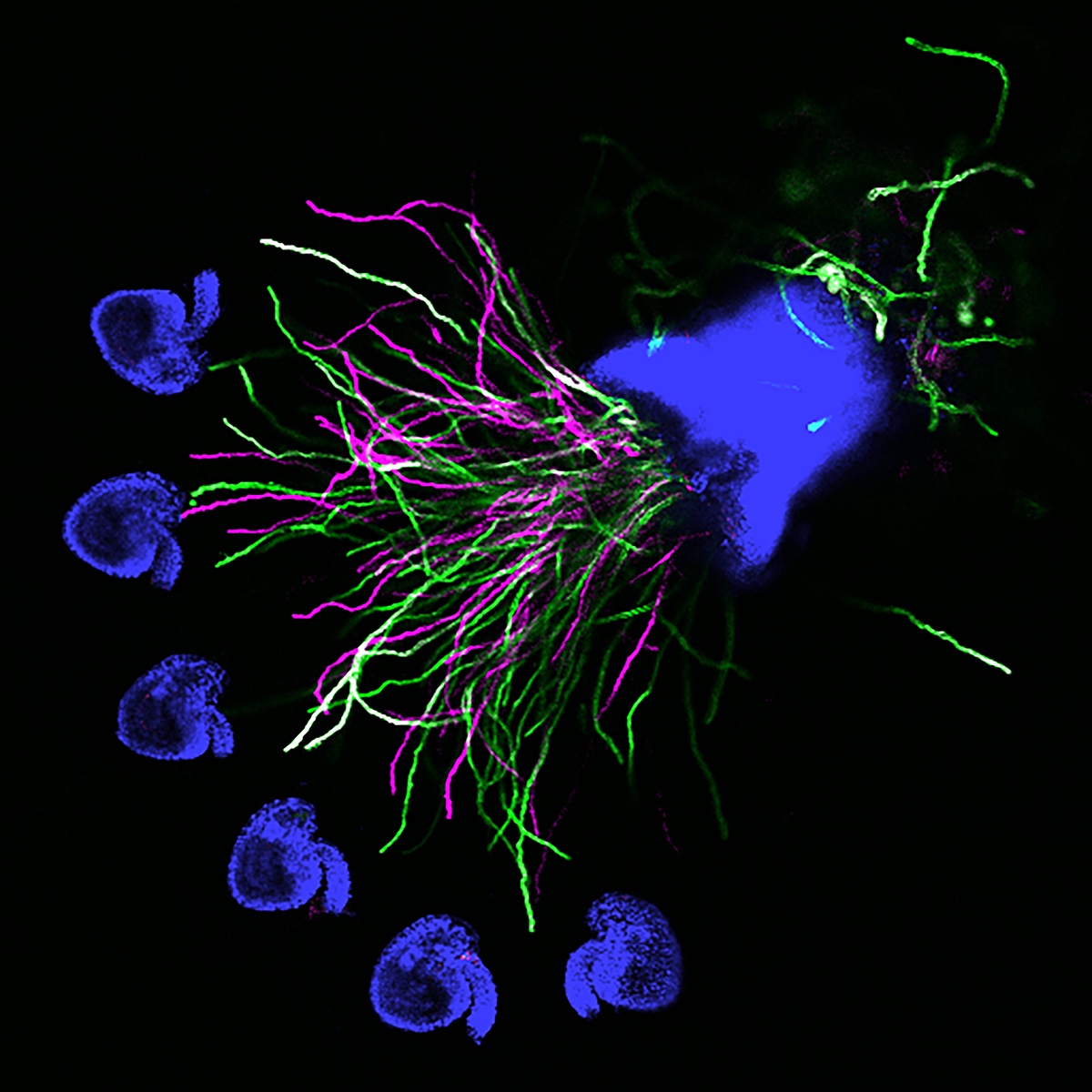
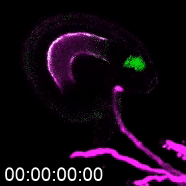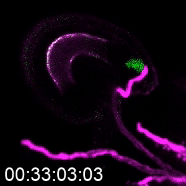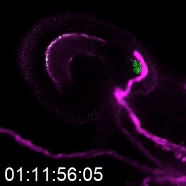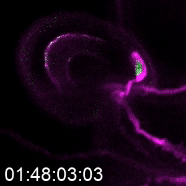
The more detailed version of plant reproduction in flowering plants works like this: In order for sperm cells to reach the ovules, pollen grains hydrate and send out a pollen tube to transport the sperm to the ovule, which will become the seed after fertilization. Kessler’s work on this aspect of plant reproduction has helped answer the question of how the pollen tube knows when to burst and release the sperm.
As a postdoctoral researcher, Kessler worked in a lab that identified a new gene involved in the signaling between the pollen tube and specialized cells in the ovule called synergids. Synergids act as the brain of the ovule, she explains, attracting the pollen tube and signaling when it’s okay for the tube to burst.
One way to determine how this communication works is to use plants with genetic mutations that disturb part of the communication process, subtracting or adding signals and observing when the pollen tube bursts and when it doesn’t. That’s how the Kessler team discovered the gene for the protein NORTIA (NTA) is part of the signaling process. NTA is part of a family of mildew-resistance proteins, which are also involved in signaling when plants are confronted with an invading pathogen. In this case, the invader — the pollen tube — is a beneficial one.
When the pollen tube arrives in the filiform apparatus, a specialized part of the synergid cell, it signals its arrival, and NTA moves from inside the cell to the filiform apparatus as well. Additional experiments showed a second protein, FERONIA (FER) is involved in signaling NTA to move and the pollen tube to burst. The Kessler lab was able to manipulate the process to determine that even if they eliminated FER, if they forced NTA to accumulate in the filiform apparatus, they could still signal the pollen tube to burst.
“It’s such an important process that the synergid really wants to make sure the pollen tube will deliver the sperm cells. So, there’s this mechanism where a back-up signaler [NTA] is called down to help. And we call this our booster-signal hypothesis. If you only have the booster, you can also make a pollen tube burst.”
Kessler likes to use an analogy to describe this signaling system. “You can think of a lot of plant reproduction as being like a lock and a key,” she says. “The female side is always the lock. The pollen side is always the key, and then the key tries to fit the lock at all of these different stages. And you’ve got to unlock this signaling pathway that then leads to the female side.”
This same lock and key system of signaling at each step of reproduction is also what prevents the wrong plant from trying to pollinate a different species. As species evolve over time, these mutations can cause a mismatch in the signaling process. Kessler’s lab has also experimented with overriding that signaling mismatch to send NTA to the filiform apparatus without the usual signal. Sometimes that NTA boost is enough to cause the pollen tube to burst, but in some cases, the species divergence is too great to circumvent the signaling system. “The maternal side doesn’t want to waste its resources on letting pollen tubes grow through where the sperm cells can’t fit,” Kessler explains.
Even more exciting, Kessler says, were hints that a similar signaling process might also occur in root hairs. As her lab also began experimenting with that pathway, they found it worked similarly. FER is usually involved in signaling, but if an NTA-like protein is transported to a specific domain in the root hair, independent of the FER signal, the root hair will still grow.
The Kessler lab’s work led to other scientists discovering that these signaling proteins act as calcium channels. They determined that you can express a plant protein in an animal cell, and it can receive a signal to traffic to the part of the cell where it can do its job — forming a channel to transport calcium, fundamental to many biological processes, into the animal cell.
“You could imagine in other cells this would also be useful,” Kessler says. “If you want to scale the amount of response that a cell is having to something in its external environment, regulation of protein trafficking to a specific part of the cell could act like a volume knob to make sure the cell’s response is correct.
She says scientists still don’t know how the calcium transported into the cell, which enters in a pattern of waves, is being interpreted to make the appropriate response. “How is all this decoded to lead to the pollen tube bursting, or to the root hair growing or to the pathogen being able to infect a cell? That’s what I’m really excited about.”
Kessler and her lab members continue to explore what all of these signals mean and how they determine what happens in plants, with funding from the National Science Foundation. “As basic scientists, we often find ourselves going in directions that we didn’t expect based on the kinds of findings that we have. We’re actually following the science.”
Following those findings leads to discoveries in applied science, too. “Applied research builds on our understanding of the basic processes that are occurring in plants,” Kessler says. For example, basic understanding of the locks and keys that control plant reproduction could lead to new strategies for creating novel crop hybrids with desirable new traits.
“What basic biologists are doing is trying to figure out mechanisms within the organism,” she says, “and then we’re publishing it so everybody can build off of each other’s results.”